The Role of Hydrogen Peroxide in Controlling Plant Cell-Signaling and Gene-Expression Patterns Related to Stress and Defense Responses
By
2014, Vol. 6 No. 06 | pg. 1/3 | »
IN THIS ARTICLE
KEYWORDS
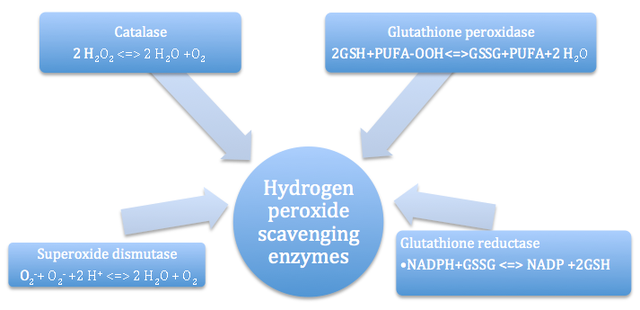
AbstractThe major purpose of this review article is to explore the influence of hydrogen peroxide on cell signaling and gene expression patterns in plants, within the context of defense responses to a variety of biotic stressors, abiotic stressors, and wounding events. Hydrogen peroxide (H2O2) is an endogenous molecule that is part of a group of cellular components referred to as reactive oxygen species (ROS) that are formed by aerobic respiration and other oxidation-related processes within the plant (Orozco-Cardenas, et al. 2001; SÌlesak et al. 2007). Majority of the early research linking H2O2 with specific plant responses to the environment dealt with responses to abiotic stressors, which are dependent on peroxide accumulation mediated by calcium ion movement and mitogen-activated protein kinase cascades. Hydrogen peroxide is also one of the earliest signaling molecules involved in wound-signaling pathways, serving as a local signal for hypersensitive cell death and cell wall stiffening events, and as a mobile signal that stimulates defense-genes in adjacent cells (Orozco-Cardenas et al. 2001). Much of the current work on this topic has focused on uncovering novel sources of H2O2 in response pathways, examining overarching molecular networks that control oxidative burst events, assessing interactions between various environmental and endogenous factors and H2O2 signaling, and studying gene products that protect cellular components during H2O2 responses. Avenues of future research are also considered, including the identification of factors with differential activity in abiotic stress versus wound-induced response pathways, applications to pest control, and creation of quantitative models for peroxide activity. BACKGROUNDThe lifespan and developmental timeframe of many plant species are characterized by a state of limited mobility. Though changes in size and growth-related parameters are normal and expected, plants lack several functional abilities as a direct consequence of their sedentary lifestyles (De-Bruxelles and Roberts 2001). In turn, this group of organisms has faced several evolutionary challenges in responding to environmental stressors and establishing defense strategies against herbivory and pathogenic attack (De-Bruxelles and Roberts 2001). However, research conducted over the last four decades has identified a set of complex defense systems against abiotic and biotic factors, involving unique molecular interactions between various compounds and genes commonly found in plant species (De-Bruxelles and Roberts 2001). One such compound that has been implicated in several pathways is hydrogen peroxide, H2O2, an endogenous molecule that is one of the most common reactive oxygen species (ROS) (Orozco-Cardenas et al. 2001). Historically, studies concerning hydrogen peroxide in plants focused on describing production sites, scavenging or removal mechanisms, and involvement in growth and development-related signaling pathways. Hydrogen peroxide and other ROS are common byproducts of normal aerobic respiration, resulting from a specific process called the disproportionation of radical superoxide, or its reduction by molecules such as ascorbate, thiols, and ferredoxins (SÌlesak et al. 2007). Within chloroplasts, hydrogen peroxide is the most abundant product of the ‘Mehler reaction’, which describes the photoreduction of O2 (Mullineaux and Karpinski 2002). Furthermore, peroxisomes serve as a major site for reactions generating H2O2 including photorespiration, β-oxidation of fatty acids, and additional oxidation of other substrates (Dat et al. 2000). Smaller levels of peroxide production also occur in the cytoplasm, plasma membrane, and the extracellular matrix of plant cells (SÌlesak et al. 2007). Along with multiple pathways for synthesis are multiple pathways for removal or scavenging of H2O2. For example, tocopheral (Vitamin E) is a lipid-soluble antioxidant that can hinder free radical reactions that result in lipid peroxidation and H2O2 generation (SÌlesak et al. 2007). Reduction of glutathione can allow it to participate in direct detoxifying reactions with ROS (Ball et al. 2004). Glutathione levels also regulate the expression of genes associated with other antioxidant defense systems in plants (Ball et al. 2004). Overall, several enzyme systems are implicated in the scavenging of peroxide and other free radicals in the cell (Figure 1). In terms of signaling during plant growth and development, hydrogen peroxide has been implicated in cell wall differentiation, stimulation of somatic embryogenesis, and induction of glutathione peroxidase and glutathione S-transferase gene expression (Levine et al. 1994; Potikha et al. 1999).Figure 1 – Flow chart of the major free radical scavenging mechanisms and reactions in plants.
Schematic diagram including the names of the specific enzymes commonly involved in removal of free radicals such as hydrogen peroxide from plants, and the specific reactions they catalyze (Modified from Quan et al. 2008). The first connections linking hydrogen peroxide with specific plant responses to the environment dealt with abiotic stressors. As one example, H2O2 was shown to mediate UV-B-induced gene expression, as indicated by down-regulation of the UV-induced gene PDF1 in Arabidopsis plants exposed to a combination of antioxidants and UV-B light (A-H-Mackerness et al. 1999). Other studies have tracked the way through which hydrogen peroxide exerts protective effects. Levine et al. (1996) introduced various stressors to soybean cells, and found that that the H2O2 generated as a result of these introductions induced rapid influx of calcium ions, a change that resulted in apoptosis. Another pathway of interest is the mitogen activated protein kinase (MAPK) cascade, which can be activated by H2O2 and subsequently translocated into the nucleus to activate specific transcription factors (Lebrun-Garcia et al. 1998). Figure 2 provides a step-by-step schematic representation of a MAPK cascade from the original signal to signal-responsive gene expression (Neill et al. 2001). In Arabidopsis species, the specific MAPK that is activated by peroxide was identified as AtMPK6, which could also be activated directly via stressors such as cold, osmotic stress, and ozone treatment (Samuel et al. 2000). Finally, Desikan et al. (2001) identified specific genes that are dependent on H2O2 for either expression or repression. cDNA microarray analyses identified 175 non-redundant sequence tags regulated by peroxide in the Arabidopsis transcriptome: 62 of these tags were repressed, while 113 were induced (Figure 3). Of the induced genes, 18% had predicted functions in cell rescue and defense processes. Figure 2 – Representation of a mitogen-activated protein kinase (MAPK) signaling cascade
Schematic diagram of a MAPK cascade including MAP kinase kinase kinase (MAPKKK), MAP kinase kinase (MAPKK), and MAP kinase (MAPK). P represents phosphorylation. T refers to threonine residues, while Y refers to tyrosine residues. (From Neill et al. 2001) Figure 3 – Functional distribution of oxidative stress-induced genes in Arabidopsis.
Pie chart representing the various functions of 113 induced genes in the Arabidopsis transcriptome (set of all RNA molecules produced) that are dependent on H2O2 for expression (From Desikan et al. 2001). Other than environmental stressors, plants have also evolved a complex set of defense mechanisms to respond to direct mechanical damage or “wounds.” Wound-signaling pathways involve the activation of many genes, both locally in damaged areas and systemically in non-damaged areas within the plant (Titarenko et al. 1997). Some of these genes include those coding for proteinase inhibitor II (Pin2 family) and vegetative storage proteins (VspA and VspB) in potato, tomato, and soybean plants (Titarenko et al. 1997). Generation of ROS, including hydrogen peroxide, is one of the early events common to many of these pathways, and is referred to as the “oxidative burst” stage (Orozco-Cardenas and Ryan 1999). H2O2 production levels reach a peak between four to six hours after wounding occurs, and then steadily decline (Orozco-Cardenas and Ryan 1999). Several genes have been found to modulate this burst, including a gene encoding the 18-amino acid polypeptide signal systemin and a gene encoding a membrane-bound NADPH oxidase complex (Orozco-Cardenas and Ryan 1999). Within wound pathways, H2O2 serves as a local signal for hypersensitive cell death and cell wall stiffening events, and as a mobile signal that stimulates defense-genes in adjacent cells (Orozco-Cardenas et al. 2001). Peroxide also has been linked to the up-regulation of phenylpropanoid genes that express lignin proteins needed for wound-sealing and formation of phenolic defense compounds (Lawton et al. 1983). RELEVANCEStudying the role of hydrogen peroxide in the context of plant defense responses is crucial for applications in crop production, general plant survival, and agricultural resource management. In terms of crop production, H2O2 can provide notable protection from herbivory of insects and other environmental causes of mechanical damage. H2O2-based signaling pathways might also play a role in guarding these plants from photooxidative damage caused by excessively high light intensities (Jänkänpää et al. 2013). In terms of basic needs for survival, hydrogen peroxide has also been associated with the stomatal closure-modulating gene, OsSRO1c, in rice (You et al. 2013). OsSRO1c overexpression promotes decreased stomatal aperture and reduced water loss, a crucial mechanism for periods of drought, along with an accumulation of H2O2 levels (You et al. 2013). Because the protection and survival of crops and other plants is crucial to various food chains and life cycles in natural environments, tracking the role of peroxides can increase understanding of controllable factors that can prevent early death and thereby increase product output. Information about endogenous hydrogen peroxide activity can also promote more efficient strategies for packaging and storage of agricultural products. One study showed that modulation of wound-induced hydrogen peroxide has an influence on survival of Escherichia coli in cut lettuce tissues (Toivonen et al. 2012). Heat-treated cut lettuce made less hydrogen peroxide and promoted increased bacterial survival compared to non-heated controls. However, when cut lettuce was treated with a fungal elicitor hairpin, H2O2 generation was enhanced and E. coli survival was reduced. Therefore, studying different modulators of peroxide action during wound responses can greatly benefit the agricultural food market, by suggesting practices that promote the durability and safety of plant products.Continued on Next Page » Suggested Reading from Inquiries Journal
Inquiries Journal provides undergraduate and graduate students around the world a platform for the wide dissemination of academic work over a range of core disciplines. Representing the work of students from hundreds of institutions around the globe, Inquiries Journal's large database of academic articles is completely free. Learn more | Blog | Submit Latest in Biology |