Featured Article:Dietary and Lifestyle Interventions to Support Functional Hypothyroidism
By
2009, Vol. 1 No. 12 | pg. 1/1
IN THIS ARTICLE
KEYWORDS
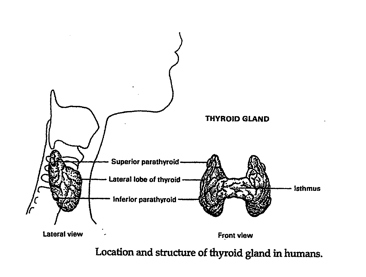
IntroductionHypothyroidism is an unsuspected illness of epidemic proportions in western civilization unrecognized by the modern medical community (Starr xix). The affects are far-reaching and pervasive. The purpose of this research is to demonstrate that addressing nutrient deficiencies, mitigating the damaging effects of the environment, and changing certain lifestyle practices can boost thyroid hormone production naturally. Research methodology includes a review of the current peer-reviewed publications by both conventional and alternative medicine practitioners, live presentations and personal interviews. Findings include a specific dietary, supplement, and lifestyle protocol or healing functional hypothyroidism developed based on the research and personal experience. Normal Thyroid FunctionThe thyroid gland is an endocrine gland located at the base of the neck comprised of reddish-brown right and left pear-shaped connected lobes weighing 10 to 20 grams. Each lobe, receiving abundant blood supply from three arteries, is divided into separate lobules. Each lobule contains between 20 and 30 million follicles responsible for the biosynthesis of thyroid hormones (Figure 1) (Norman, et al. 171).
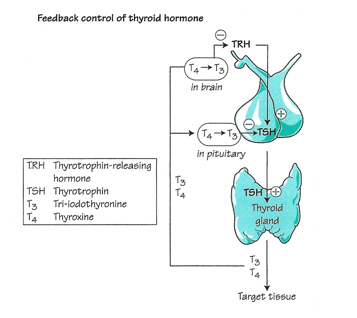
Thryoid hormones are chemical messengers made of proteins that interact with internalized hormone receptors at the cellular level to sustain a specific biological. Thyroid hormones are synthesized as a result of a negative feedback loop that involves the pituitary gland and thyroid-stimulating hormone (TSH) or thyrotropin, and the hypothalamus and thyrotropin-releasing hormone (TRH) (Figure 2). The biosynthesis of thyroid hormones requires thyroglobulin (Tg) the most abundant protein in thyroid tissue, thyroid peroxidase (TPO), the key enzyme in the synthesis of organically bound iodine, H2O2 (peroxide), and iodine from the diet (Norman et al. 172, 177). This negative feedback loop governs iodine uptake and the synthesis of Thyroxine (T4) by the thyroid gland. TSH is the most critical hormone affecting iodine uptake (Liska et al. 175, Norman Litwack 177).
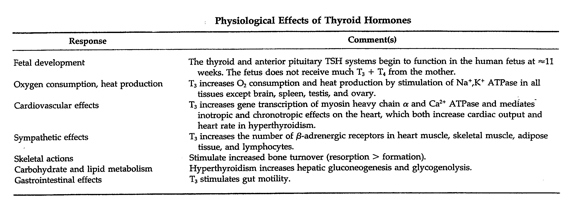
The physiological regulation of the secretion of thyroid hormones by the thyroid gland via this negative feedback loop depends on blood concentration of T4 and triiodothyronine (T3), the two primary thyroid hormones which regulate the pituitary secretion of TSH. Thyroid hormones are released into the blood by hormone-producing cells in the follicles in response to a change in the concentration of thyroid hormones in the blood. These actions are orchestrated by polypeptide TSH receptors located in the cell membranes of the thyroid follicle cells. T4 is the chief thyroid hormone secreted by the thyroid gland. Approximately 80% of the T4 secreted by the thyroid gland is converted to equal amount of T3 and reverse T3 (rT3) in peripheral target cells, in the liver and in the kidneys, but the concentration of T4 in the blood is 40 to 100 times greater than that of T3 (ATA 24 May 2005, Hedberg June 2009, Liska et al. 175, Norman and Litwack. 180-1, Milner 2, Paoletti 4). T3 is the principal source of energy for our cells. rT3, the inactive form of T3, can bind to T3 receptor cells and as an antagonist of T3, can inhibit the activity of T3, even when T3 levels are optimal – rT3 needs to be displaced by the biologically active T3 to normalize metabolism (Milner 2, Paoletti 4). The actions of various T3 receptors located in the nucleus of peripheral cells throughout the body mediate a wide variety of biological responses (Norman and Litwack 185, 187). (Table 1). (No T4 receptors have been identified in the body (Paoletti 4).) Approximately 90% of T3 binds with these intracellular thyroid receptors (Starr, 243). When peripheral cell receptors capture these “messages” (the mechanism is analogous to a key fitting into a lock) the cell responds to the presence of the hormone. Table 1. Reprinted from Hormones, 2nd Ed., Norman, Anthony and Gerald Litwak, Chapter 6, Page 185, Copyright 1997, with permission from Elsevier.
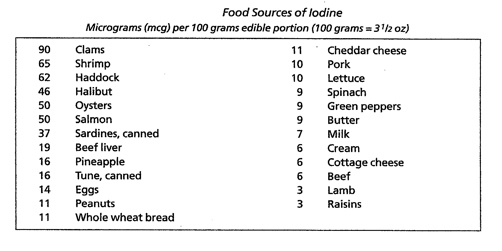
Thyroid hormones regulate all metabolic activities – growth rate, sodium/potassium pump, cholesterol secretion in the bile, heart rate, heart strength, blood pressure, respiration, oxygen consumption (basal metabolic rate (BMR)), digestion, lipid, carbohydrate and protein metabolism, central nervous system function, and the actions of other endocrine glands. Normal sexual function is dependent on normal thyroid secretion. Low levels of thyroid hormones can have effects on behavior, growth, cardiac output, GI function, tissue oxygen consumption, muscle strength, and immune function. (Liska et al. 175, Norman and Litwack 169, 171, Starr, 243, 247-8). Characteristics of HypothyroidismHypothyroidism (type 1) is defined as “. . . failure of the thyroid gland to produce sufficient amounts of thyroid hormones necessary to maintain ‘normal’ blood levels of thyroid stimulating hormone (TSH). . . marked by lowered basal metabolism, subnormal body temperature, and myxedema . . .” (edema resulting from the abnormal accumulation of mucin)(Starr 255-7). Major symptoms include enlarged heart, lowered cardiac output, cool skin, reduced sweating, poor appetite, reduced GI activity, enlarged skeletal muscles with myopathy (weakness), and a drooping upper eyelid (Norman and Litwack 172, 189). Below normal thyroid hormone levels can have dramatic effects and may be life threatening (Standard in Natural Solutions). In 2007 it was estimated that approximately two percent of the U.S. population, or 5.8 million people, are hypothyroid. Women are more likely to develop hypothyroidism than men and the incidence increases with age. As many as 24% of women over sixty are hypothyroid. The Colorado Thyroid Disease Prevalence Study (1995) found that approximately 10% of the general population suffered from untreated (functional or subclinical) hypothyroidism. As many as 10 to 15% of Americans have mild or subclinical hypothyroidism and may not know it. Even with the more narrow range of the standard TSH test, approximately 13 million Americans with thyroid disease remain undiagnosed today. (ATA 2003, 7, Brady 2, Milner 2, Murray “Hair Loss in Women” 1712, Paoletti 3,Teitelbaum 121-2). Family history is the greatest risk factor for hypothyroidism, according to the American Thyroid Association (ATA), but others include those of older age, especially women whose risk increases if they are white or Asian and have reached menopause, having another autoimmune disorder, Down syndrome, Turner’s syndrome, or bipolar disorder. Conditions that increase risk include thyroid surgery, radiation to the neck area, and medicines that can cause hypothyroidism, including amiodarone, lithium, interferon alpha, interleukin-2, and thalidomide. (2003 at 7,8). Definition of Functional HypothyroidismFunctional hypothyroidism has been defined as a state where blood levels of thyroid hormone fall within normal range, but where temperature tests and other indicators show mild thyroid hormone deficiency (Standard in Natural Solutions). Even mild thyroid hormone deficiency can have far-reaching effects. Sometimes referred to as subclinical hypothyroidism, functional hypothyroidism has been characterized by optimal levels of TSH and T4 with the presence of symptoms (Paoletti 4), or by slight to moderately elevated TSH levels with normal levels of T4 (Murray and Bongiorno “Hypothyroidism” 1795, Nadeau 90). The ATA defines subclinical hypothyroidism as mild subclinical hypothyroidism with normal T4 and slightly high TSH (TSH in the range of 4-10 mU/L) and subclinical hypothyroidism (TSH >10 mU/L) that may cause mild symptoms or none at all (2003 at 3, 2004). In comparison, clinical hypothyroidism is characterized by abnormal levels of thyroid hormones due to failure of the thyroid gland. Causes include loss of functional thyroid tissue1, mild to severe autoimmune conditions, genetics and congenital factors, nutrient deficiencies2, exposure to toxins, failure in the hypothalamus-pituitary-thyroid (HPT) axis, and stress (Murray and Bongiorno “Hypothyroidism” 1794-5, Norman and Litwack 189, Paoletti 3, Fisher et al. 2722). Persons with breast cancer may develop hypothyroidism as a result of radiation treatments (ATA 2008, 2009). The ATA defines hypothyroidism as either primary, a failure of the thyroid gland because of a condition directly affecting the thyroid gland, or secondary hypothyroidism, a rare condition resulting from an abnormality in the pituitary gland (ATA 2005). In either case it is a permanent condition. Type 2 Hypothyroidism or Resistance to Thyroid HormoneDr. Mark Starr defines Type 2 hypothyroidism as peripheral resistance to thyroid hormones (RTH) at the cellular level from inherited defective mitochondria, resulting in a decline of cellular energy in tissues of the central nervous system, heart and skeletal muscle, kidneys, and hormone-producing tissues. Thyroid hormone resistance may be an autoimmune condition caused by inherited mutations of thyroid hormone receptor genes resulting in inappropriate treatment in one-third of cases. Persons with thyroid hormone resistance as a result of pituitary tumors may be mildly hypothyroid, i.e. show normal or slightly increased TSH. There are no blood tests to detect peripheral RTH. (Arafah and Nasrallah 299, Norman and Litwack 189, Olateju and Vanderpump 431, Shoman 2, Starr, 45, 167.) A person can suffer from both type 1 and type 2 hypothyroidism (Starr 53) and persons treated with thyroid hormone replacement therapy, either synthetic or desiccated thyroid hormone, may experience only partial or temporary relief of symptoms but show optimal blood levels of hormones (Paoletti 3, Milner 1). In 1996 Dr. Martin Milner began monitoring symptoms of persons taking levothyroxine and though their doses were adequate based on serum testing, more than 75% continued to suffer from symptoms of hypothyroidism (2). Wilson’s SyndromeWilson’s Syndrome, or Wilson’s Temperature Syndrome, is a condition characterized by deficient peripheral conversion of T4 to T3 and excessive peripheral conversion to the inactive form, rT3, with symptoms of low thyroid function, low BMR, and low body temperature (below 98.6 º) given normal thyroid (TSH) tests (ATA 24 May 2005, Murray and Bongiorno “Hypothyroidism” 1792, Shomon 4, In-Tele-Health). The ATA has found no scientific evidence for the existence of Wilson’s syndrome (Ibid). Etiology of Functional Hypothyroidism ToxinsFunctional hypothyroidism can be caused or exacerbated by environmental toxins. Environmental toxins interfere with normal cellular function. Antibody reactions and toxicity due to heavy metals or environmental pollutants adversely affect the conversion of T4 to T3 (Paoletti 5). A vast number of chemicals have been shown to interfere with the production, transport and metabolism of thyroid hormone (Appendix A). In addition processed foods exposed to herbicides, pesticides, fumigants, food additives, acrylics, fungicides, and pthalates adversely affect thyroid hormone metabolism. Contaminants found in chlorinated city water, including trihalomethane, benzene, arsenic, and halogens, such as bromide, fluoride and iodide, can interfere with thyroid hormone function (Hedberg June 2009, Paoletti 3). Dioxins, BPA, mercury in dental amalgams and other heavy metals interfere with the actions of thyroid hormones. Polychlorinated biphenyls, PCBs, and polybrominated diphenyl ethers resemble thyroid hormones in structure and can interfere with the HPT axis. Triclosan, found in hand sanitizers, and Splenda can interfere with thyroid hormone function (Hedberg June 2009). The thyroid gland is the main target organ for perchlorate, a chemical used in rocket fuel, explosives, and pyrotechnics, which in high doses can inhibit thyroid hormone production (Pirkle). NutritionNutrition plays a critical role in thyroid hormone synthesis. The production of thyroid hormones depends on adequate amounts of tyrosine, a non-essential amino acid synthesized in the body from the essential amino acid phenylalanine, and dietary iodine. Tyrosine may be conditionally essential in the absence of phenylalanine (Whitney and Rolfes 183). Iodine is reduced to organically bound iodine in the intestines. Approximately 90 to 95% of all available iodine in the body is located in the thyroid gland (Norman and Litwack 172). T4 contains 4 atoms of organically bound iodine and T3 contains three atoms of organically bound iodine. The RDA for iodine is 150 mcg per day according to the US National Research Council (Norman and Litwack 170). But individual needs for iodine varies (Williams 157). Iodine deficiency is the most common cause of hypothyroidism worldwide, but it is rare in the U.S. according to the ATA (2003 at 6). We get iodine from foods (Table 2) and it is found in commercially processed iodized salt and preservatives used in bread products. (Natural unrefined sea salt is not a good source of iodine – ¼ tsp of sea salt contains 15 mcg, less than 10% of the RDA for iodine.) Excess iodine can inhibit thyroid hormone production by increasing cellular resistance to T3 and may lead to iodine-induced thyroid deficiency, swelling, tenderness and goiter (Brinker 127, Hedberg April 2009m Shomon 7). Table 2. Reprinted from Clinical Nutrition, A Functional Approach, Liska, DeAnn, Ed. et al., Chapter 6, Page176, Copyright 2004, with permission from The Institute for Functional Medicine.
Soy and seaweeds may decrease the absorption of levothyroxine (Synthroid) (Rybacki 1306). Drugs that may interfere with the absorption of levothyroxine include antacids containing aluminum hydroxide, bile acid sequestrants, calcium carbonate, and ferrous sulfate (Ananthakrishnan and Pearce 27). Some foods contain substances known as goitrogens that interfere with the body’s ability to use iodine. Goitrogens can induce iodine deficiencies by binding to iodine and making it unavailable (Appendix B). Cyanogenic compounds, found in cruciferous vegetables, flax seeds and soy isoflavones, particularly genistein, break down to isothiocyanates. Isothiocyanates decrease thyroid function by blocking thyroid peroxidase, the enzyme responsible for transporting iodine into the cell. Fermentation may reduce these effects, especially if eaten with foods high in iodine, and may even increase the levels of B vitamins, particularly B6 (Kohlstadt 112, Daniel 49). L-carnitine is an antagonist of thyroid hormone in peripheral tissues – it can prevent it’s entry into the cell (Murray and Bongiorno “Hyperthyroidism” 1775). Excessive exogenous hormone may suppress the production of endogenous hormone. The proper balance of T3 and rT3 is dependent on adequate selenium. Conversion depends on the selenium-dependent enzyme 5′ deiodinase (Milner 4). Selenium binds to mercury and protects the thyroid gland against toxins. Selenium is deficient in about 50% of the population and selenium deficiency can degrade cellular thyroid activity, even though hormone levels are normal (Murray and Bongiorno “Hypothyroidism” 1795). Selenium content in foods depends on the selenium content of the soil where those foods were grown (Milner 4). Vitamin C enhances the absorption of selenium (Lieberman 217). Adequate iron is also required for thyroid metabolism, as are zinc and copper (Liska et al. 175-6). Vegetarian diets are high in copper, which can contribute to zinc deficiency (Billica and Willner). Zinc also plays a role in ridding the body of excess estrogens, balances adrenals, and lowers stress (Hedberg April 2009). Alcohol, diuretics and oral contraceptives interfere with zinc absorption (Ibid.). The late Dr. Shari Lieberman advises to make note of the amount of elemental zinc in our supplements (190). Vitamin A, Vitamin C, Vitamin D, Vitamin E, bioflavonoids, B vitamins, and balanced essential fatty acids (2:1 to 1:1, Omega 6 to Omega 3) are required for optimal thyroid hormone production. Vitamin E, selenium, zinc, and Vitamin B6 enhance the conversion process. Vitamin A optimizes receptor sites. B vitamins, cofactors in enzyme metabolism required for every bodily function, help the liver metabolize estrogen, balance hormones, and reduce the effects of stress. B vitamins, especially Vitamin B12 and folic acid, are depleted by mercury and environmental toxins. Hypochlorhydria reduces our ability to assimilate B vitamins, especially B12. Vegetarian diets are lacking in usable B12 and B6 (found only in animal foods). Sugar contributes to B vitamin deficiencies. Vitamin C is an iodine transporter and protects against free radical damage caused by stress. Vitamins A and D support immunity and, therefore, the thyroid gland (Hedberg April 2009, Fallon 38, Kitchen, Liska et al. 175-6, Shames and Shames 156-60, Shomon 4, 7, Vanderhaeghe 88, 95). Potassium is important for proper functional of the adrenal glands. Potassium is required for conversion of blood sugar into glycogen (Murray, Encyclopedia 179) and adrenal hormones can be depleted as a result of low levels. Most fruits and vegetables will shift the balance of K:Na in favor of potassium. Suboptimal levels of Vitamin D and ferritin, high or low levels of cortisole, and dysfunction of cell receptors can cause thyroid hormone resistance, as can excessive amounts of essential (Omega 6) fatty acids, vitamin D or progesterone. (Paoletti 6). One study showed reduced incidence of hypothyroidism with supplemental Vitamin A in Vitamin A and iodine-deficient children in developing countries, suggesting that Vitamin A supplementation improves iodine efficacy (Zimmerman et al. 5441). OthersImbalances in digestive function, overactive immune system, blood sugar dysregulation, adrenal malfunction, food allergies and sensitivities, particularly to gluten, and poor detoxification processes can upset optimal thyroid health (Hedberg April, June 2009, Kharrazian 88, Shames and Shames 9). Most of thyroid hormones, 80%, are transported in the blood bound by thyroxine-binding globulin (TBG). The remaining small amounts of unbound hormones are significantly affected by changes in TBG levels and affect the amount of active hormones available to the cells. Functional hypothyroidism can be caused by excessive binding through increased TBG caused by excessive circulating estrogen. TBG levels are increased by oral thyroid replacement, oral estrogens, and pregnancy (Paoletti 5), as well as alcohol consumption. Stress has a negative impact on thyroid hormone production. Excess cortisol inhibits the production of 5′ deiodinase, decreases peripheral T3 receptors, and increases the production of rT3 (Hedberg April 2009, Paoletti 3, Brady 4 )3. Tyrosine prevents excessive rises in cortisol levels (Paul) and has been used therapeutically in the treatment of depression (Braverman et al. 39). Infection compromises the adrenals and the immune system, and therefore, thyroid function. Wilson’s Syndrome may be a function of stress (Shomon 4). Acute critical illness can cause a type of functional hypothyroidism in which there is a brief rise in TSH with normal levels of T4, but low levels of T3 (Mechanick 588-90). Adrenal insufficiency and low levels of cholesterol contribute to inadequate sex hormones, which can compound low thyroid conditions (Shames and Shames 39, Hedberg June 2009). Hormonal changes such as puberty, perimenopause and menopause stress the adrenal glands (Shomon 4). Insulin is the chief hormone regulating intermediary metabolism in which proteins, carbohydrates and fats are utilized by the metabolic processes involved in glucose homeostasis, the delicate balance throughout the body that results in either mobilization of stored glucose, glycogen, or storage of excess glucose as glycogen in response to changes in calorie intake and degree of physical activity (Norman and Litwack 194 – 221). Since this mechanism is responsible for mobilizing fat as an energy source for muscles and the brain, amino acid uptake into skeletal muscle, and the incorporation of amino acids into proteins by the liver, insulin resistance can affect this balance and contribute to nutrient deficiencies (Greenstein and Wood 83). Tests, Diagnosis and TreatmentConventional Approach The ATA does not recognize the existence of functional hypothyroidism, nor does the ATA believe that low body temperature, slow reflexes, saliva tests, or swelling in the base of the neck are valid assessments for physicians to use in diagnosing hypothyroidism. The ATA recommends that adults, especially women, be screened for thyroid dysfunction every five years beginning at age 35, and that individuals with symptoms of potential thyroid dysfunction, and persons at risk for it’s development, may require more frequent testing. Physicians are advised to look at the patient’s symptoms, medical history, and risk factors, such as family history. Radioactive iodine uptake, RAIU, or thyroid scan may be used to detect abnormalities. The best way to test thyroid function is to measure the serum levels of thyroid hormones. Primary hypothyroidism is characterized by High levels of TSH and low levels of T4. Secondary hypothyroidism is characterized by low levels of TSH and T4. Physicians may also look at fT4 or the fT4 index (total T4 plus thyroid hormone binding ratio). Testing of rT3 is rarely helpful in the hypothyroid persons. (2003 at 10, 2005, Higgins, Ladenson et al. 1573.) The reference value for normal thyroid output (TSH) was very broad prior to 2003 (Table 3) and many people who would have shown normal levels may have been deficient (Shames and Shames 17). The new guidelines for TSH optimization adopted in 2003 provide a new range for TSH between .3 to 3.0 µIU/ml. Table 3. Reference Ranges for thyroid hormones prior to 2003.
The ATA recommends testing for thyroid antibodies at TSH levels greater than 2.5 mU/L and considering treatment for subclinical hypothyroidism at levels above 4.0 mU/L (2003 at 9). Conventional treatment for subclinical or mild hypothyroidism may include administration with T4, levothyroxine, a bioidentical synthetic hormone that must be converted in the liver and kidneys to T3. Brand names include Synthroid®, Levothroid®, Levoxyl®, and Unithroid®. The ATA recommends brand name drugs over generics and that once thyroid function has been stabilized, persons remain on the same brand (2003 at 14). Excessive thyroxine can cause bone loss, weaken muscles and cause serious heart trouble (ATA 2003 at 17). Synthetic T3 (Cytomel and Thyrolar) is four times more biologically active than T4 and exerts its effects more rapidly and vigorously. Persons are more likely to experience side effects from synthetic T3 than from compounded slow released T3, including tachycardia, arrhythmia, anxiety, nervousness, agitation, irritability, sweating, headaches, increased bowel motility, menstrual irregularities, and aggravation of conditions such as angina, congestive heart failure, and atrial fibrillation (Milner 3). Combination T4 and T3 is not advocated in all except persons who have had their thyroid gland surgically removed (ATA 2003 at 16). The ATA does not advocate the use of thyroid glandular extracts because products are not purified, the balance of T4 to T3 is not natural to humans, and levels of hormones may vary from batch to batch. Armour Thyroid tablets, USP, which contain a ratio of four parts of T4 to one part of T3, comparable to those produced by the human and porcine thyroid glands, meet standards established by the United States Pharmaocopeia (USP), undergo bacteriological testing, and tests to ensure that the levels of T4 and T3 are the same from tablet to tablet (Forest Laboratories, Inc.). The ATA believes synthetic thyroxine is safer and maintains that Chinese herbs, selenium, tyrosine, kelp supplements, and other herbal remedies won’t work once the thyroid gland stops doing its job. Alternative Approach The alternative approach is to look at symptoms, related conditions, family history and physical signs. Signs include fatigue, lethargy, sleepiness, mental impairment, depression, cold intolerance, slow movements and speech, inadequate reflex response, hoarseness, dry skin, decreased perspiration, weight gain, decreased appetite, constipation, joint pain, slow heart rate, dry skin, myxedema, paresthesia, diminished or delayed reflexes, shortness of breath, impaired kidney function, loss of libido in men and menstrual disturbances. Hair loss in women is a cardinal sign of hypothyroidism but hair loss can be attributed to other conditions. (Murray “Hair Loss in Women” 1712, Murray and Bongiorno “Hypothyroidism” 1792-3, Starr 19). Fatigue and depression are generally the first clinical signs, with difficulty concentrating and forgetfulness developing later as the condition progresses (Murray and Bongiorno “Hypothyroidism” 1793. According to Dr. Martin Milner, “The most common complaints include fatigue, impaired concentration, and persistent difficulty losing weight in spite of adequate exercise and reasonable caloric restrictions.” (1). Because typical blood tests measure TSH and T4, if peripheral conversion from T4 to T3 is not taking place a person may have normal blood levels of thyroid hormone and still be deficient (Murray and Bongiorno “Hypothyroidism” 1792). Before conventional blood tests became the norm, assessment for hypothyroidism was based on a measurement of the BMR and Achilles reflex response. The axillary (armpit) basal body temperature test, a functional test developed by the late Dr. Broda Barnes, measures the resting BMR, and therefore, the effect of thyroid hormones on the body. The generally accepted temperature range for normal thyroid hormone function is between 97.8 to 98.2º F. When Dr. Milner’s patients were asked to record their first morning axillary basal body temperature, they revealed values consistently below 98º F (2). Many factors can affect the basal body temperature (Murray and Bongiorno “Hypothyroidism” 1794). Unless the reading is consistently below normal, a sluggish metabolism may not be a consideration – how you feel may be the most accurate assessment (Shames and Shames 52-4). Alternative medicine practitioners may also use conventional serum thyroid hormone levels to assess thyroid function. The rT3-to-T3 ratio is used to assess Wilson’s Syndrome (Murray and Bongiorno “Hypothyroidism” 1792). Jim Paoletti compares fT4 to fT3 (free T3) to determine if a patient is converting normally and recommends assessing adrenal function with saliva testing four times a day. Michael Murray and Peter Bongiorno recommend low-dose thyroid hormone supplementation in persons with TSH greater than 2.5 µIU/ml if alternative intervention is not successful (“Hypothyroidism” 1794). Persons with RTH may have varied serum hormone levels with elevated fT4 and fT3, normal-to-slightly elevated TSH, a goiter, but no symptoms (Olateju and Vanderpump 431). Supporting metabolism with proper nutrients, instead of increasing T4, is recommended when the problem is converting T4 to T3 (Hedberg April 2009). Because serum tests may miss cases of mild hypothyroidism, and because adrenal and thyroid function are interdependent, urinary excretion of thyroid and adrenal metabolites and electrolytes may be used to assess thyroid and adrenal function (Brady 3). The TRH test is believed to be more sensitive in assessing subclinical hypothyroidism and has been found useful in assessing hypothalamic dysfunction as a factor in affective disorders (Murray and Bongiorno “Affective Disorders” 1430, Hedberg June 2009.) Still other experts believe in simply looking at symptoms of adrenal exhaustion, especially hypoglycemia (Wilson 59, Teitelbaum 123). Prescription desiccated thyroid hormone extract from porcine sources, such as Armour Thyroid, Naturethroid, and Westhroid, is typically used in conjunction with nutritional and supplement protocols and lifestyle interventions. The belief is that these are less toxic and provide a better clinical and symptomatic response than synthetic compounds, particularly in persons having difficulty converting T4 to T3 (Brady 5). In many cases thyroid hormone replacement may not be necessary (Kharrazian 89). Practitioners may recommend supplemental T3 thyroid hormone for person’s with Wilson’s Syndrome. Excessive thyroid glandular concentrates may trigger autoimmune thyroid disorders (Brady 6) and, because the T3 is released immediately, may cause side effects, including antioxidant depletion, anxiety, tremor and palpitations (Murray and Bongiorno “Hypothyroidism” 1797). Some experts recommend using compounded slow release T3 in combination with T4, while monitoring serum hormone levels (Milner 7), and lowering T4 gradually (Paoletti 4, 7). To support the health of a person with symptoms of functional hypothyroidism the diet should include optimal nutrients for thyroid hormone production and peripheral conversion from T4 to T3 and to reduce or eliminate receptor site resistance (Shoman 2). Specific nutrients to include are L-tyrosine, iodine, selenium, zinc, Vitamin E, B vitamins and over-the-counter thyroid extracts (in which most of the T3 has been removed) (Brady 4-5). A diet high in organic whole foods and fiber will help eliminate heavy metals and other toxins from the body. Fermented foods augment intestinal bacteria, increase the production of B vitamins in the intestine, and normalize stomach acid secretion (Fallon 38, 101). Adequate sources of cholesterol will help to balance sex hormone levels. Avoid consumption of conventionally raised animals. These animals are fed hormones, antibiotics and pesticide-laden GMO feed which disrupt natural hormone production and are harmful to humans and to the environment. Avoid aspartame, which is metabolized to phenylalanine, a potential neurotoxin, aspartic acid, which can cause brain damage, and methanol, which the body converts to toxic formaldehyde. Commercially processed iodized salt contains aluminum and sugar and can be difficult to metabolize by the human body. (Shomon 2, 4). Dr. Edward Bauman, who frequently works with patients who are on thyroid hormone replacement, finds that patients who support thyroid function nutritionally for several months are then in a position to consider tapering down their medication dosage over time. He suggests using dulse from hand harvested non-commercial sources, rather than kelp, for organically bound iodine and trace elements better suited nutritionally for the thyroid gland. Chlorella, a single-celled algae high in trace elements, amino acids, and omega 3 fatty acids, can help aid in detoxification. Coconut oil is a source of butyric acid, which helps the transport of T3 into the brain, and short and medium chain fatty acids, which help to modulate blood sugar and protect the mitochondria from stress. He recommends adequate protein from a plant-based, but not necessarily vegetarian, diet and gentle massage of the thyroid gland. (Shomon 4-5,7). Coconut oil, iodine and zinc can increase the BMR (In-Tele-Health). Avoiding or eliminating toxins from pesticides, synthetic chemicals, heavy metals, and tap water is paramount. Dental mercury is in close proximity to the thyroid and is toxic, even in minute amounts – oral chelation therapy using nutrients and herbs is always recommended (Shomon 3). Assess and address adrenal insufficiencies and develop methods for avoiding or reducing stress before attempting to resolve thyroid problems (Shomon 4, Paoletti 7). Botanicals known to be helpful in balancing thyroid hormones may be helpful with functional hypothyroidism: ashwaganda supports thyroid hormone production; Oregon grape, B. aquifolium, stimulates the thyroid gland (Ritchason 160); gentian, G. lutea, has a normalizing effect on the thyroid gland (Ritchason 96); bee propolis is an antimicrobial that stimulate immunity and boosts thyroid function (Ritchason 22); nettle, U. dioica, is a source of iron and potassium for cellular homeostasis and support of thyroid hormone production and the adrenals; parsley, P. sativum, has good amounts of Vitamin A, Vitamin C, copper and manganese, nutrients that support thyroid hormone production (R. Wood 247, Ritchason 163); black walnut, juglans nigra, is a thyroid gland stimulant high in iodine (Karstens); chickweed, stellaria media, is useful when TSH levels are elevated, but T3 and T4 are below normal; and bladderwrack, is a source of iodine to help normalize hormone production. (Shomon 7, M. Wood 56-7). Adaptogenic herbs, such as rhodiola, R. rosea, and Siberian ginseng, E. senticosus, normalize endocrine function (Hedberg, June 2009, Murray and Pizzorno “Eleutherococcus Senticosus” 921, Ritchason 104, Shackelton) and have been shown to increase a general sense of well-being (Murray and Pizzorno “Stress Management” 707). Siberian ginseng may have mild side effects if taken in large doses for longer than 60 days and may potentially be contraindicated in hypertensive persons (Brinker 86, Murray and Bongiorno “Eleutherococcus Senticosus” 923). Even though side effects are less likely than with other types of ginseng, prolonged use without periodic breaks is not recommended. Associated Diseases and DisordersFunctional hypothyroidism may affect the function of all body systems. Dr. Mark Starr has revisited the work done by Dr. Barnes, who previously concluded that type 2 hypothyroidism is a causative factor in heart disease, autoimmune disorders, mental and neurological disease, chronic fatigue syndrome, high blood pressure, anemia, poor circulation, kidney disease, dizziness and vertigo, TMJ Syndrome, diseases of the digestive tract, eating disorders, MS, cancer, hypoglycemia, and disease of the gallbladder and bladder. Functional hypothyroidism may also be implicated in Meniere’s disease, menstrual disorders, problems associated with infertility and menopause, headaches and migraines, chronic pain, arthritis and fibromyalgia, obesity, and liver disease. Low thyroid hormone levels affect the face, skin, hair, nails, eyes, hearing, teeth, gums, swallowing, the tongue, speech, and body weight. Low thyroid hormone levels can exacerbate other conditions, including anemia, inflammation, difficulty sleeping, fatigue, CFS, malnutrition, parasites, hyperthyroidism, toxicity, Lyme disease, allergy, lack of exercise and depression. These same conditions can make it difficult to assess a low thyroid condition as many conditions may coexist with low thyroid hormone output (Shames and Shames 22, 39, 47). A low T4 level is correlated with mortality in critically ill patients (Mechanick 599). Research shows that thyroid hormones preserve cardiac function, help to prevent CVD, and that low levels increase the risk of CVD (In-Tele-Health). Low thyroid hormone levels have been shown to increase plasma concentration of cholesterol, phospholipids and triglycerides, homocysteine and C-reactive Protein, causing severe arteriosclerosis, decreased cardiac output and function (aortic stiffness), and hypertension (ATA 2002, Murray and Bongiorno “Hypothyroidism” 1793, Starr, 246). Results of a six month study showed that hypothyroidism, even at the subclinical level, is associated with increased risk for cardiac disease (Anderson 63). In a press release dated October 1, 2004, the ATA announced that individuals with subclinical hypothyroidism have twice the risk of developing heart disease compared with those with normal levels of TSH. However, proper thyroid supplementation is believed to prevent heart attacks (Starr 34-35). Functional hypothyroidism can present similar clinical symptoms of and may be an underlying cause of fibromyalgia (Schneider and Brady 534, Hedberg Sept 2009). Hypothyroidism is a factor in affective disorders and depression (Murray and Bongiorno “Affective Disorders” 1431). One half of subjects with RTH hormones have some degree of learning disability, with or with out attention deficit hyperactivity disorder, ADHD (Olateju and Vanderpump 434). Mild, subclinical, hypothyroidism has been associated with higher incidence of atrial fibrillation in older persons, reduced bone mineral density, particularly in postmenopausal women, and palpitations (ATA “Screening for Thyroid Dysfunction”). There is a link between men with hypothyroidism and erectile dysfunction (ATA 2009). Findings and ConclusionFunctional hypothyroidism, caused by faulty metabolism of T4 to T3 that results in an excess of rT3, or thyroid hormone resistance at the cellular level due to inherited defective mitochondrial or thyroid hormone receptor genes, is a function of diet and environmental factors. Persons may not be experiencing the major clinical signs of hypothyroidism, but may show more subtle signs of fatigue, impaired concentration, and persistent difficulty losing weight, which may make functional hypothyroidism difficult to assess. Functional hypothyrodism is distinguished from clinical hypothyroidism by the absence of abnormal blood tests. When blood levels of TSH and T4 are “normal”, symptoms of functional hypothyroidism may persist since normal conversion to T3 is assumed. The absence of adequate biologically active T3 thyroid hormone, despite an adequate level of T4 and TSH, can cause symptoms of hypothyroidism – serum levels of fT3 and rT3 may be normal when T3 and rT3 are imbalanced. The present reference ranges for serum levels of thyroid hormone are narrower than they were before 2003, however this simply served to increase the number of persons diagnosed with thyroid disease. Many persons with “normal” serum levels of thyroid hormones continue to experience symptoms. The standard TSH test can still miss hypothalamic hypothyroidism, suppressed TRH production, common in fibromyalgia and affective disorders (Murray and Bongiorno “Affective Disorders” 143, Teitelbaum 121), and doesn’t take into consideration biochemical individuality. There is lack of agreement about classification of types of hypothyroidism. Even given the new lower ranges, the upper limit of TSH, and therefore “normal” serum levels, has been the subject of considerable controversy. Consequently no normal TSH range limits have been determined and uniformly applied clinically. It remains a matter of interpretation, leading to confusion about what constitutes subclinical or functional hypothyroidism, and many persons remain undiagnosed and untreated. Functional hypothyroidism contributes to the risk for CVD and may cause learning disabilities. It has been associated with atrial fibrillation in older persons, reduced bone mineral density, palpitations, and can potentially exacerbate or complicate many other conditions. (Fibromyalgia and affective disorders may be manifestations of suppressed TRH production, rather than functional hypothyroidism.) The ATA maintains that there is no evidence in support of thyroid hormone resistance (2003 at 7). Conventional treatment is generally not recommended unless TSH is greater than 10 µIU/ml. Historically experts haven’t agreed on whether or not subclinical hypothyroidism should be treated – some treated only those with symptoms. In 2003 the ATA claimed that more research was needed. It wasn’t until October of 2004, after the publication of a study linking subclinical hypothyroidism to CVD, that the ATA recognized the necessity to treat subclinical hypothyroidism (TSH >10 mU/L). Experts agree, to some extent, that nutrition plays a critical role. The ATA maintains, as do alternative practitioners, that iodine, either too much or too little, can affect thyroid hormone function, but disagrees, unlike alternative practitioners, that soy and other goitrogens can cause hypothyroidism, or that supplemental key nutrients can restore thyroid function. Alternative practitioners focus on 1) supporting the health of and balancing all the body systems, 2) supporting proper conversion of T4 to T3, 3) avoiding and eliminating toxins, and 4) and mitigating factors, physical and environmental, that cause stress. Alternative practitioners may combine conventional serum tests with less conventional tests to assess thyroid hormone function, or simply look at symptoms. Many have found that compounded slow release T3 used in combination with T4 can help to mitigate many of the symptoms of functional hypothyroidism and improve the quality of life in persons suffering from these symptoms. Still others, by supporting the thyroid with proper nutrients, obviate the need for supplemental hormones. The use of T3 alone is not an accepted treatment by most practitioners, conventional or alternative, but slow-release T3 may be recommended. Regardless of the methods employed, diet plays a key role. Because blood tests do not pinpoint functional hypothyroidism, and because the axillary temperature test isn’t always accurate, assessments, such as questionnaires and saliva tests, and food journals will be useful to the clinical nutrition consultant. Often it is going to be simply a matter of how the client feels. Thyroid Health-Promoting ProtocolAssessments will be useful in pinpointing functional hypothyroidism. Focus on total body health, digestive health, medical history, hypoglycemia, and thyroid self assessment. Have amalgams removed one at a time followed by a three-day minimum amalgam removal detoxification protocol. Address digestive issues, particularly hypochlorhydria. Address any chronic or acute infections, particularly candida. Consider an elimination diet to rule out food allergies and food insensitivities, especially to gluten. Assess adrenal insufficiency. If adrenal fatigue is likely, confirm using saliva tests. If the assessment indicates mild fatigue, support with whole foods, otherwise consider a glandular (not suitable for vegans and vegetarians). The axillary temperature that measures the BMR may not be accurate, especially when using a digital thermometer. Advise clients to keep a diary of foods and beverages consumed, physical activity, basal body temperature, and to note how they feel. The diet should be a plant based, whole foods diet that is high in complete, biologically available protein, and optimum levels of vitamins, minerals, antioxidants, phytonutrients, and complex carbohydrates. It should include organic free range and grass fed meat, poultry and dairy products, wild-caught cold water fish (favor those species at the bottom of the food chain, such as bristling sardines, herring, and mackerel), organic fresh whole grains, fruits and vegetables, and leafy greens. Keeping the diet alkaline, supporting digestion, supporting the liver, especially if thyroxine is being taken, supplying adequate soluble fiber, regulating blood glucose levels, and eliminating toxins will support the health of the thyroid gland. The following protocol can be adapted to meet individual biochemical needs. Recommend a few things at a time and introduce new foods gradually, making use of the food journal to gauge results. • Support digestion. Avoid drinking beverages for 15 minutes before and after eating. Eat without distraction in a relaxed manner and chew food thoroughly. This engages the parasympathetic nervous system and aids digestion. Include cultured and fermented foods for beneficial bacteria. Goat dairy may be easier to digest. Gentian, in addition to stimulating the secretion of digestive juices, has the added benefit of normalizing thyroid hormone production and being an antimicrobial. Apple cider vinegar before meals will stimulate the secretion of stomach acid. Supplement with digestive enzymes. Lacto-fermenting legumes before cooking predigests the sugars, making them easier to digest, and lacto-fermented foods increase stomach acid secretion. • Avoid or reduce exposure to environmental toxins. Eat organic foods only and avoid exposure to toxins from yard chemicals, household cleaners, personal hygiene products, plastics, and other chemicals. Home Safe Home by Debra Lynn Dadd is a valuable resource. Toxins disrupt thyroid hormone production, but indoles found in cruciferous vegetables will support the liver and help it to detoxify the body. Eggs provide sulfur-containing amino acids methionine and cysteine, and molybdenum, a mineral coenzyme, all required for the liver's detoxification pathways. Plant foods high in Vitamin C and soluble fiber support detoxification. Herbal teas support the liver and gallbladder, key organs of detoxification. • Water eight to ten eight-ounce glasses. Drink filtered water only. (See www.mercola.com.) Carry a stainless steel water bottle filled with water you have filtered yourself. • An alkaline diet supports health. Replace trace minerals in filtered water with Concentrace® and unrefined sea salt. Vegetable and bone broths are good sources of trace minerals, required for thyroid hormone production and activation of thyroid hormone. Fresh vegetable juices with a big handful of fresh parsley will support the thyroid. Herbal teas, dandelion root and leaf and nettle, will aid in detoxification. • Eat moderate amounts of high quality protein with each meal and snack. Protein is required for the production of thyroid hormone and liver detoxification. Best sources are from meat, poultry, sea foods, and dairy products. These are also the best sources of zinc and selenium, minerals required for thyroid hormone production. The protein in an egg is considered by experts to be 100% biologically available, but the protein in properly processed whey protein powder is as much as 159% biologically available (Null and Martin 66, In-Tele-Health “Whey Protein”). Plant sources, though complete, may not be as biologically available. Spirulina and nutritional yeast may be acceptable sources of protein for vegans – consider also supplementing with a complete amino acid supplement and an additional multi-mineral formula. • Eat Foods high in Soluble Fiber to help balance blood sugar. Fresh whole fruits and vegetables, leafy greens, nuts and seeds, and whole grains are good sources of soluble fiber, which can help regulate blood sugar levels. • Supplement with EPA and DHA fish oils. Many health conditions may be attributed to essential fatty acid deficiencies. Essential fatty acids are required constituents of every membrane in the body. The body uses ALA for many purposes. But current research indicates that as little as 5% may be available for conversion to EPA and then to DHA (In-Tele-Health Path: “Alpha-Linolenic Acid” “Conversion”). Ultra purified fish oil (3 g/day independently tested for rancidity, heavy metals, dioxins and PCB’s) are the best sources of EPA and DHA. Spirulina may be an acceptable source of EPA and DHA for ovo-lacto vegetarians and vegans. • Pre-formed Vitamin A supports thyroid function. Persons with low thyroid function because of a zinc or vitamin E deficiency may have trouble converting beta carotene, found in brightly colored vegetables and fruits, to preformed Vitamin A. (Murray, Encyclopedia 19, 22.) Cod liver oil is a source of vitamins A and D, supportive of optimal thyroid hormone function. • Exercise regularly and avoid stress. Stress can contribute to elevated blood sugar levels. In addition to helping to manage stress, exercise increases thyroid hormone production and tissue sensitivity. Yoga poses, shoulder stand for example, stimulate the thyroid gland. Deep breathing and yoga engage the parasympathetic nervous system. Adaptogenic herbs enhance long-term ability to cope with physical and environmental stressors. Specific nutrients, such as B vitamins and zinc, help mitigate the effects of stress. • Don’t diet. Dieting slows metabolism. • Aromatherapy. Clary sage helps to balance hormones. • Supplement Recommendations. Supplements may include a multivitamin mineral with antioxidants, additional selenium, Vitamin C, and Vitamin E , a high potency B complex, sublingual Vitamin B12, digestive bitters, EPA/DHA fish oil, digestive enzymes, probiotics, and free-form amino acids. Consult with a licensed health care practitioner before taking supplements. Foods to Include Organic foods are higher in nutrients required to support the thyroid gland, including the trace minerals zinc, copper, iodine, manganese and selenium, Vitamin A, Vitamin C, Vitamin D, Vitamin E, potassium and the amino acids tyrosine and phenylalanine. Good sources include meat, liver, seafood, especially shrimp and oysters, poultry, especially dark meat, eggs, dairy products, especially butter, beans and legumes, especially garbanzo beans, whole grains, especially buckwheat, amaranth, quinoa, brown rice and oats, apples, strawberries, pears, pineapple, kiwi fruit, apricots, spinach, romaine, cinnamon, black pepper, turmeric, parsley, goat milk kefir and yogurt, Brussels sprouts, broccoli, cabbage, cauliflower, red and orange bell peppers, sweet potatoes, citrus fruits, acerola berries, fresh dandelion (leaf and roots), dandelion leaf and root tea, coconut oil, crimini mushrooms, nuts and seeds, especially unshelled Brazil nuts and pumpkin seeds, walnuts, almonds, almond butter, ground flax seeds (small amounts), sesame and hemp seeds, avocados, spirulina, chlorella, dulse, cod liver oil, nutritional yeast, blackstrap molasses, eggs, whey protein, miso, tempeh, sauerkraut and umeboshi. Protein sources for vegans will include legumes, passion flower, almonds, mustard seeds, fennel, garlic, bananas, spirulina, kale and natto. Foods and substances to limit or avoid Limit refined carbohydrates, including sugar, and high glycemic foods. These can raise blood glucose levels. Elevated blood sugar contributes to insulin resistance, which can inhibit the passage of nutrients into the cell. Limit fruit to two whole a day and choose low glycemic sources (see foods to include). Avoid excessive fiber from supplements such as psyllium and wheat, as these can bind to available thyroid hormone. Some foods contain goitrogens and other substances that can interfere with the body’s ability make thyroid hormones. Goitrogenic foods include broccoli, Brussels sprouts, cabbage, cassava root, cauliflower, collard greens, flax seeds, horseradish, kale, kohlrabi, mustard greens and seeds, millet, peaches, peanuts, pine nuts, radishes, rape seeds (canola oil), rutabaga, soybeans and soy products, spinach, strawberries, and turnips. These substances are generally inactivated during cooking. Limit to less than one serving cooked per day and one cup raw per week. Refined and processed foods are sources of oxidized cholesterol, hidden sugar, trans fats, excess sodium, excess amounts of Omega 6 fats, and artificial sweeteners, all of which can interfere with nutrient absorption and promote inflammation and oxidative damage to tissues. For good health balance Omega 6 to Omega 3 fats in a ratio of 2:1 or 1:1. Hemp seeds provide a good balance of Omega 6 to Omega 3 fats. Include sources of healthy cholesterol to support synthesis of sex hormones from eggs, butter, dairy, and shrimp. Iodine and tyrosine, a non-essential amino acid, are required for the cellular conversion of T4 to T3, the active hormone. Excessive iodine can depress thyroid hormone production and conversion to the active form, especially in persons taking synthetic T4. High-iodine intake has been implicated as a cause of hypothyroidism in one case study (Werbach 217). Fast foods contain excessive amounts of iodine (Dunne 39). Limit consumption of iodine to food sources and don’t consume in excess. Choose low heat processed sources of protein. The protein in hydrolyzed whey protein and plant-based protein powders can be denatured (damaged) and processed whey protein frequently contains additives. Avoid alcohol, as it can contribute to decreased thyroid hormone production and slow metabolism. Tap water contains chemicals that are toxic to the thyroid gland. References 1. American Thyroid Association. “American Thyroid Association Statement on ‘Wilson’s Syndrome’.” 24 May 2005. thyroid.org. 19 Oct. 2009 . 2. American Thyroid Association. “Thyroid Function Tests.” 2005. thyroid.org. 19 Oct. 2009 . 3. American Thyroid Association. “Thyroid Problems Increase Risk of Heart Disease and Death.” ATA News Release. 01 Oct 2004. thyroid.org. 24 Sept. 2009 . 4. American Thyroid Association. “ATA Hypothyroidism Booklet.” 2003. thyroid.org. 24 Sept. 2009. . 5. American Thyroid Association. “Thyroid and Erectile Dysfunction.” Clinical Thyroidology for Patients. 1:1. (2008). 30 Sept 2009 < http://www.thyroid.org/patients/notes/aug08/08_08_22.html>. 6. American Thyroid Association. “Thyroid Hormone.” Clinical Thyroidology for Patients. 2:1. (2009). 30 Sept 2009 < http://thyroid.org/patients/ct/volume2/issue1/ct_patients_v21_3.html>. 7. American Thyroid Association. “Hypothyroidism.” Clinical Thyroidology for Patients. 2:3. (2009). 30 Sept 2009 < http://thyroid.org/patients/ct/volume2/issue3/ct_patients_v23_5.html>. 8. Ananthakrishanan, Sonia and Elizabeth Pearce. “Hypothyroidism and Hyperthyroidism: Review Questions.” Hospital Physician. May 2007. 24 Aug 2009 . 9. Anderson, Robert. “Psychoneruoimmunoendocrinology Review and Commentary.” Townsend Letter. 305 (2008): 62-65. 10. Arafah, B.M. and M.P. Nasrallah. “Pituitary tumors: pathophysiology, clinical manifestations and management.” Endocrine-Related Cancer. 8 (2001): 287-305. 11. Billica, Roger and Catherine Willner. “Molecules of Behavior II: Diagnosis & Treatment of Neurotransmitter Imbalances.” Designs for Health, Inc. 21 June 2008. 12. Brady, David. “Functional Thyroid Disorders, Part I.” Dynamic Chiropractic. 18:7 (2000). 13. Braverman, Eric, et al. The Healing Nutrients Within. Laguna Beach: Basic Health Publications, Inc., 2003. 14. Brinker, Francis. Herb Contraindications & Drug Interactions. 3rd Ed. Sandy, OR: Eclectic Medical Publications, 2001. 15. Cowan, Tom. “Ask the Doctor About Hypothyroidism.” 31 Mar. 2001. westonaprice.org. 29 Aug. 2007 < http://www.westonaprice.org/askdoctor/hypothyroidism.html>. 16. Daniel, Kaayla. The Whole Soy Story, the dark side of America’s favorite health food. Washington, D.C.: New Trends Publishing, 2007. 17. Dunne, Lavon. Nutrition Almanac. 5th Ed. New York: McGraw-Hill, 2002. 18. Fallon, Sally and Mary Enig. Nourishing Traditions. Rev. 2nd Ed. Washington, DC: New Trends Publishing, 2001. 19. Fisher, D. A. et al. “The Hypothalamic-Pituitary-Thyroid Negative Feedback Control Axis in Children with Treated Congenital Hypothyroidism.” Journal of Clinical Endocrinology & Metabolism. 85:8. (2000): 2722-27. 20. Forest Laboratories, Inc. “Frequently Asked Questions.” 2009. armourthyroid.com. 11 Apr. 2008 < http://www.armourthyroid.com/con_faqs.aspx>. 21. Greenstein, Ben and Diana Wood. The Endocrine System at a Glance. 2 Ed. Malden, MA: Blackwell Publishing, 2006. 22. Hedberg, Nicholas. “Understanding Thyroid Imbalances.” Hawthorn University Live Webinar. 28 April 2009. 23. Hedberg, Nikolas. “Understanding Thyroid Imbalances, Part 2.” Hawthorn University Live Webinar. 09 June 2009. 24. Hedberg, Nikolas. “Fibromyalgia.” Hawthorn University Live Webinar. 29 Sept 2009. 25. Higgins, Thomas. “Thyroid Function Tests.” 2006. bouldermedicalcenter.com. 28 Oct. 2009 . 26. Hollander, Jason, and Jeffrey Mechanick. “Nutrition Support and the Chronic Critical Illness Syndrome. Nutrition in Clinical Practice. 21. (2006): 587-604. 27. Howdeshell, Kembra. “Endocrine Disruptors. A Model of the Development of the Brain as a Construct of the Thyroid System.” Environmental Health Perspectives. 110:3. (2002): 337-48. 28. HyperHealth Pro v. 8.0 – Encyclopedia of Nutrition and Natural Health. CD-ROM. Hansville, WA: In-Tele-Health, 2008. 29. Karstens, Connie. Personal Interview. 30 March 2009. 30. Kharrazian, Datis. “The Real Cause of Hypothyroidism.” Townsend Letter. 316 (2009): 88-9. 31. Kitchen, Judy. “Hyphchlorhydria: A Review, part 1.” Townsend Letter for Doctors and Patients. 219-221. (2001). 13 Sept 2009 . 32. Kohlstadt, Ingrid. “Hypothyroidism: More Seaweed, Less Soy, and a Closer, Potentially Life-Saving Look at Iodine.” Townsend Letter. 305 (2008): 62-65. 33. Krohn, Jacqueline and Frances Taylor. Natural Detoxification. Pt. Roberts, WA: Hartley and Marks Publishers Inc., 2000. 34. Ladenson, Paul, et al. “American Thyroid Association Guidelines for Detection of Thyroid Dysfunction.” Archives of Internal Medicine. 160 (2000): 1373-75. 35. Lieberman, Shari. The Real Vitamin & Mineral Book. 3rd Ed. New York: Avery, 2003.
37. Liska, DeAnn, Ed., et al. Clinical Nutrition, A Functional Approach. Gig Harbor: The Institute for Functional Medicine, 2004. 38. Mateljan, George. The World’s Healthiest Foods. 1st Ed. Seattle: George Mateljan Foundation, 2007. 39. McDermott, Michael and E. Ridgway. “Subclinical Hypothyroidism Is Mild Thyroid Failure and Should be Treated.” 2001. jcem.endojournals.org. 14 July 2009 < http://jcem.endojournals.org>. 40. Milner, Martin. “Hypothyroidism: Optimizing Medication with Slow-Release Compounded Thyroid Replacement.” Townsend Letter. Feb/Mar 2007. 30 July 2009 . 41. Murray, Michael. Encyclopedia of Nutritional Supplements. N.Y.: Random House, 2001. 42. Murray, Michael. Total Body Tune-Up. New York: Bantam Books, 2000. 43. Murray, Michael and Peter Bongiorno. “Eleutherococcus Senticosus.” Ed. Pizzorno, Joseph and Murray, Michael. Textbook of Natural Medicine. 3rd Ed. New York: Churchill Livingstone, 2006. 44. Murray, Michael. “Hair Loss in Women.” Ed. Pizzorno, Joseph and Murray, Michael. Textbook of Natural Medicine. 3rd Ed. New York: Churchill Livingstone, 2006. 45. Murray, Michael and Peter Bongiorno. “Hypothyroidism.” Ed. Pizzorno, Joseph and Murray, Michael. Textbook of Natural Medicine. 3rd Ed. New York: Churchill Livingstone, 2006. 46. Murray, Michael and Peter Bongiorno. “Hyperthyroidism.” Ed. Pizzorno, Joseph and Murray, Michael. Textbook of Natural Medicine. 3rd Ed. New York: Churchill Livingstone, 2006. 47. Murray, Michael and Peter Bongiorno. “Affective Disorders.” Ed. Pizzorno, Joseph and Murray, Michael. Textbook of Natural Medicine. 3rd Ed. New York: Churchill Livingstone, 2006. 48. Murray, Michael and Joseph Pizzorno. “Stress Management.” Ed. Pizzorno, Joseph and Murray, Michael. Textbook of Natural Medicine. 3rd Ed. New York: Churchill Livingstone, 2006. 49. Nadeau, Andre. “Subclinical Thyroid Disease: A laboratory Finding of Clinical Relevance?” Canadian Journal of CME. July 2001: 89-98. 50. Norman, Anthony and Gerald Litwack. Hormones. 2nd Ed. New York: Academic Press, 1997. 51. Null, Gary and Martin Feldman. “The Argument for a Vegetarian Diet, Part One.” Townsend Letter. 310 (2009): 63-68. 52. Olateju, Tolulope and Mark Vanderpump. “Thyroid Hormone Resistance.” Annals of Clinical Biochemistry. 43 (2006): 431-40. 53. Paoletti, Jim. “Hypothyroidism, Functional Hypothyroidism, and Functional Metabolism.” International Journal of Pharmaceutical Compounding. 12:6 (2008): 489-97. 54. Panaretou, Dernellis. “Effects of thyroid replacement therapy on arterial blood pressure in patients with hypertension and hypothyroidism.” Am Heart J. 143. (2002): 718-24. 55. Paul, Cristiana. “L-Tyrosine. What we Should Know About This Important Amino Acid.” Product Tech Sheet. designsforhealth.com. 2009. 56. Pirkle, James. “CDC’s Perchlorate Biomonitoring Activities and Study Results.” CDC Congressional Testimony before the Committee on Energy and Commerce Subcommittee on Environment and Hazardous Materials, United States House of Rep. 25 Aug 2007 < http://www.cdc.gov/search.do?queryText=perchlorate&action=search>. 57. “ Reference Ranges for Blood Tests.” Wikipedia, the Free Encyclopedia. (28 Oct. 2009). Wikimedia Foundation, Inc. 08 Oct 2009 < http://en.wikipedia.org/wiki/Reference_ranges_for_blood_tests#Hormones> . 58. Ritchason, Jack. The Little Herb Encyclopeids. 3rd Ed. Pleasant Grove, Utah: Woodland Health Books, 1995. 59. Rybacki, James. The Essential Guide to Prescription Drugs 2006. New York: HarperCollins, 2006. 60. Schmid, Ronald. Traditional Foods Are Your Best Medicine. Rochester, VT: Healing Arts Press, 1997. 61. Shackelton, Mary. Personal Interview. 02 July 2007. 62. Shames, Richard and Karilee Shames. Thyroid Power. 1st Ed. New York: HarperCollins, 2001. 63. Shoman, Mary. “The Metabolic Detective: A Look at Nutrition for Your Thyroid, Interview with Dr. Edward Bauman.” 11 Nov. 2007. thyroid-info.com. 01 Nov. 2009 . 64. Standard in Natural Solutions, LLC. “An Encyclopedia of Natural Medicine.” HealthQuest Database. CD-ROM. Ft. Collins, CO: HealthQuest, Inc., 2004. 65. Schneider, Michael, and David Brady. “Fibromyalgia Syndrome: A New Paradigm for Differential Diagnosis and Treatment.” Journal of Manipulative and Physiological Therapeutics. 24:8. (2001): 530-41. 66. Starr, Mark. Hypothyroidism Type 2: The Epidemic. Columbia: Mark Starr Trust, 2009. 67. Teitelbaum, Jacob. “Pain Free 1-2-3! Treat the Patient – Not the Blood Tests!” Townsend Letter. 306. (2009): 121-4. 68. “Thyroxine.” Wikipedia, the Free Encyclopedia. (31 Oct. 2009). Wikimedia Foundation, Inc. 26 June 2008 . 69. United States Department of Health and Human Services, Public Health Services, Agency for Toxic Substances and Disease Registry. “ToxGuide® for Perchlorate and Perchlorate Salts.” 14 July 2009 . 70. Vanderhaege, Lorna. Healthy Immunity. New York. Kensington Publishing: 2001.ypothyroidism Type 2: The E pidemic.ICcCC`CColum 71. Venes, Donald, Ed. et al. Taber’s Cyclopedic Medical Dictionary. 20th Ed. Philadelphia: F.A. Davis Company, 2005. 72. Werbach, Melvin. Case Studies in Natural Medicine. Tarzana, CA: Third Line Press, 2002. 73. Whitney, Eleanor and Sharon Rolfes. Understanding Nutrition. 9th edition. Belmont, CA: Thompson-Wadsworth, 2005. 74. Williams, Roger. Biochemical Individuality. New Canaan, CN: Keats, 1998. 75. Wilson, James. Adrenal Fatigue. The 21st Century Stress Syndrome. Petaluma, CA: Smart Publications, 2001. 76. Wood, Matthew. Excerpt from unpublished book. Handbook of Traditional Western Herbalism, Vol II. Minnestra, Mn: 2004. 77. Wood, Rebecca. The New Whole Foods Encyclopedia. New York: Penguin Books, 1999. 78. Yarnell, Eric and Kathy Abascal. “Urtica dioica (Stinging Nettle).” Ed. Pizzorno, Joseph and Murray, Michael. Textbook of Natural Medicine. 3rd Ed. New York: Churchill Livingstone, 2006. 79. Zimmerman, Michael, et al. “The Effects of Vitamin A Deficiency and Vitamin A Supplementation on Thyroid Function in Goitrous Children.” Journal of Clinical Endocrinology and Metabolism. 89:11. (2004): 5441-7. 1.) As in chronic autoimmune thyroiditis (Hashimoto’s disease), postradioactive iodine treatment (used to treat Grave’s disease), and thyroidectomy. 2.) Iodine deficiency can still occur in the U.S. in areas where the soil is iodine-depleted (Paoletti 3). 3.) Excess cortisol also inhibits the production of TSH, a factor in clinical hypothyroidism. 4.) Milli-International Units per milliliter.
The use of terms that describe the methods used by licensed healthcare practitioners to diagnose and treat thyroid disorders in no way implies, nor is the use of these terms to be interpreted to mean, that nutrition consultants diagnose or treat disease, patients, disorders or symptoms. Suggested Reading from Inquiries Journal
Inquiries Journal provides undergraduate and graduate students around the world a platform for the wide dissemination of academic work over a range of core disciplines. Representing the work of students from hundreds of institutions around the globe, Inquiries Journal's large database of academic articles is completely free. Learn more | Blog | Submit Latest in Health Science |