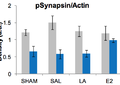
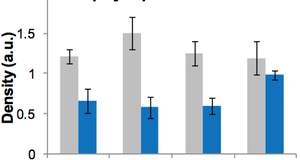
Featured Article:Recent Advances in Neural Stem Cell Research: How Stem Cells in the Brain Are Altered by a Changing Environment
By
2010, Vol. 2 No. 03 | pg. 1/1 Introduction to NeurogenesisThe discovery of adult neurogenesis (the endogenous production of new neurons) in the mammalian brain more than 40 years ago (Malcolm R. Alison, 2002) has resulted in a wealth of knowledge of this branch of neuroscience. Today we know that the continuous production of new neurons is facilitated by adult neural stem or progenitor cells (NSC/NPCs) (Cattaneo & McKay, 1990; Gage, 2000; Temple, 2001). These are self renewing, multipotent cells which possess the capability to differentiate into any neural cell type by asymmetric cell division (Temple, 2001). Our current knowledge indicates that the production of new cells in the brain follows a multi-step process during which newborn cells are submitted to various regulatory factors that influence cell proliferation, maturation, fate determination and survival. Progenitor cells isolated from the forebrain can differentiate into neurons in vitro, as was demonstrated by Reynolds and Weiss in 1992 (Gage, 2000). Since then NSCs have been isolated from various areas of the adult brain, including non-neurogenic areas such as the spinal cord. Today we know that there are two discrete regions - the Subgranular Zone (SGZ) of the dentate gyrus and the Subventricular Zone (SVZ) of the lateral ventricle, where the production of migrating neuroblasts occurs (Emsley, Mitchell, Kempermann, & Macklis, 2005; Gage, 2002). Fortunately, the migration of new born cells is not limited to these areas. The discovery of neurogenesis in the adult human hippocampus in 1998 (Eriksson et al., 1998) has offered a tantalizing possibility to medical science: that endogenous progenitor cells might be manipulated to provide a neuronal replacement therapy for brain diseases (Lovell-Badge, 2001) (McKay, 2004). Given the neurogenic property of the SVZ, it is essential to examine to what extent it responds to in a changing environment, such as that harboured by disease or injury, in ischemia and the chronic neurodegerative disease of Alzheimer’s disease (AD) for example. Here we review briefly some key studies which establish that, far from being dormant tissues, the neurogenic regions of the brain respond to neurodegerative disease in a way which makes them potential targets for therapeutic intervention (Maurice A. Curtis, Faull, & Eriksson, 2007).Effects of a Changing Environment: Neurodegenerative DiseaseDespite earlier attempts to restore function in neurodegenerative disorders via the application of cell-replacement strategies based on intracerebral transplantation of pre-differentiated embryonic progenitors in vitro (Temple, 2001), a fundamental tenet remained unanswered: does neuronal replacement from endogenous precursors occur in the brain in response to a toxic insult? A fundamental piece of this puzzle has been to elucidate whether or not new neurons migrate to the site of injury to replace those cells which have died. There are now several studies characterising the response of the SVZ and SGZ to conditions of disease (Arvidsson, Collin, Kirik, Kokaia, & Lindvall, 2002; Maurice A. Curtis, Eriksson, & Faull, 2007; M. A. Curtis et al., 2005; Jin, Galvan et al., 2004; Tattersfield et al., 2004). Neuronal replacement from endogenous precursors in the adult brain after stroke (Arvidsson et al., 2002) The pioneering study in 2002 led by Arvidsson and colleagues was the first to comprehensively show that new neurons have the capability to replace cells lost at the site of an insult. In their study Arvidsson et al hypothesised that new neurons were able to be generated in the adult rat striatum following Middle Cerebral Artery Occlusion (MCAO), a lesion model for stroke (Gao, Liu, Lu, Xiang, & Wang, 2006; Yanamoto et al., 2003). The resulting infarct depicted an almost complete loss of NeuN (marker of mature neurons) cells in the ipsilateral striatum (ST). Animals were injected with bromo-2’-deoxyuridine (BrdU), a thymidine analogue incorporated into DNA during cell division, to label and identify dividing cells. An immunohistochemical approach was used to label migratory immature and mature neurons, using Doublecortin (DCX) and NeuN, respectively. Upon analysis, MCAO was found to stimulate neurogenesis in the damaged ST at 5 weeks post MCAO, as depicted by a 31 fold increase in co-expression of BrdU + and NeuN+ cells at the ipsilateral ST, compared to contralateral and sham controls. Upon examination of BrdU+ immunoreactivity in the SVZ at 4 weeks post MCAO, there was a significant increase in the number of BrdU+ cells in ipsilateral brains as compared to contralateral and sham control. This was in accordance with the hypothesis that the newly generated cells at the ST originated from the SVZ. Interestingly, the resulting marked reduction of BrdU-Dcx+ cells in the ipsilesional ST following administration with the anti-mitotic agent, Ara-C, confirmed that the increase in newly born cells at the ST was a result of increased cell proliferation and recruitment of neuroblasts. Finally, confocal microscopy at the level of the ST 2 weeks post MCAO depicted the co-localisation of DCX with Meis2 (a transcription factor expressed in ST precursors), and Hu (an early neuronal marker), with a concomitant lack of co-localisation with the glial markers GFAP and Vimentin. BrdU cells also expressed DARPP32, a neurotransmitter expressed in MSSN neurons, cells which are selectively lost in stroke. Taken together, the experiments above showed that cortical injury resulted in the up regulation of endogenous progenitor cells, as well as the migration and neuronal differentiation at the site of cell death (ST). Unfortunately the apparent regeneration of new neurons only accounted for 0.2% of the lost striatal neurons (Arvidsson et al., 2002). Recently studies have examined the functional integration of disease-induced generation of new neurons (Lan Zhang et al., 2007; Thored et al., 2006; Yamashita et al., 2006), to establish whether a new population of cells has functional significance at the site of injury. Using immunohistochemical methods, Yamashita et al traced the migration of neuroblasts from the SVZ to ST, demonstrating a chain like migration, associated with astrocytes and blood vessels as well as formation of synapses with neighboring striatal cells(Yamashita et al., 2006). Recent studies have also focused on the presence of chemokines and attractants for injury induced migration (Barkho et al., 2006) (Thored et al., 2007). For example, Barkho et al have studied the identification of astrocyte-expressed factors that may modulate neural stem proliferation, where they demonstrated that the presence of astrocytes in neurogenic regions promoted neurogenesis. In contrast, astrocytes from non-neurogenic regions inhibited neurogenesis (Barkho et al., 2006). In addition, recent evidence for stroke induced neurogenesis in the adult human brain has emerged, providing substantial support for the potential of a neurogenic therapy to target cell loss(Jin, Wang et al., 2006). Overall the study led by Arvidsson has been instrumental in initiating the field of endogenous stem cell repair in response to CNS injury. Increased hippocampal neurogenesis in Alzheimer’s disease (AD) (Jin, Peel et al., 2004) Despite the myriad research involved in elucidating the pathophysiology of AD, a progressive neurodegenerative disease typified by senile plaques, composed of B-amyloid (AB) and the presence of neurofibrillary tangles (NFT), composed of hyper-phosphorylated tau protein, there is presently no effective treatment for AD. The establishment that AB protein disrupts neurogenesis in the SVZ and hippocampus in mouse models of AD (N. J. Haughey et al., 2002) has stimulated interest of whether this is replicated in human post mortem tissue. As a result, Jin et al examined endogenous neurogenesis in the hippocampus to verify the conflicting effects of AD pathology on the rat and human brain. The expression of immature neuronal markers, which signal the birth of new neurons, was studied in both AD and control hippocampi. Hippocampal western blotting was carried out to detect and identify the following markers: doublecortin (DCX - a microtubule associated marker located in somata and processes of migrating and differentiating neurons), polysialylated neural cell adhesion molecule (PSA-NCAM - a plasma membrane glycoprotein expressed by neuronal progenitors and differentiating neurons and astroglia in response to several different toxic insults), TUC-4 (a marker expressed early in neuronal differentiation in the rat), NeuroD (a basic helix-loop-helix protein expressed during terminal differentiation), NeuN, Calbindin, and actin, the latter to serve as a control. The resulting banding pattern (i.e. the increased expression of DCX, PSA-NCAM, TUC-4, and NeuroD) in contrast to the stagnant expression of Calbindin and NeuN, both markers for mature neurons, suggested that neurogenesis might be enhanced in the hippocampus of AD patients. Jin et al also demonstrated immunohistochemical evidence for increased neurogenesis in the hippocampi of AD brains, with TUC-4+ and DCX+ cells being localised both to the SGZ and GCL compared to control, which only labelled cells in the SGZ. In particular, compared to healthy control hippocampi, DCX+ cells were identified in the CA1 region of the diseased brains, providing strong evidence for the recruitment of new neurons to a region which is the primary site of hippocampal pathology in AD (Jin, Peel et al., 2004) and one which experiences the most severe neuronal loss. Finally, it was established using double labelling Immunohistochemistry with Hu and PSA-NCAM, that the newly generated TUC-4+ and DCX+ cells were of a neuronal lineage, and not simply expressing characteristic neurogenic markers. With the resulting increase in expression of neuronal markers in the hippocampi of AD brains and immunocytochemical localisation to known sites of neurogenesis (DG) and AD pathology (CA1), these findings are consistent with earlier studies of increased neurogenesis in response to epilepsy and stroke (Arvidsson et al., 2002; Crespel et al., 2005; Jin, Wang et al., 2006). Consequently, this study has had important implications for hippocampal neurogenesis in response to a chronic neurodegerative insult such as AD, where the hippocampus is selectively and disproportionately targeted. The fact that there exits an apparent compensatory mechanism even in response to AD, a condition which occurs at increasing frequency with advancing age adds support that this inherent plasticity of the brain can be manipulated (Maurice A. Curtis, Faull et al., 2007; Jin, Peel et al., 2004). Indeed, the recapitulation of hippocampal neurogenesis in an animal model of AD (Jin, Galvan et al., 2004) lends substantial evidence of the hippocampus as a potential therapeutic target. Although no present evidence connects neurogenesis with improved function or slower disease progression in AD (Jin, Peel et al., 2004), there is speculation that NMDA receptor inhibition to enhance dentate neurogenesis might contribute to the antagonists of NDMA receptors such as Memantine, which are currently in use to alleviate AD symptomatology in patients (Jin, Xie, Mao, & Greenberg, 2006). In light of this however, the culmination of evidence from several studies which show an overall down regulation of neurogenesis, with AB impairing proliferation and neuronal differentiation of cultured human and rodent progenitor cells (Haughey, Liu, Nath, Borchard, & Mattson, 2002), means the implications from such studies are somewhat conflicting for AD. Despite this, the demonstration by Becker and colleagues, that immunotherapy against the N terminus of AB stimulated endogenous neurogenesis (Becker, Lavie, & Solomon, 2007), i.e. the accumulation of BrdU by a high number of cells and subsequent co-labelling with NeuN (a marker for mature neurons) is suggestive of anti-amyloid immunotherapy promoting recovery of AB toxicity by partial restoration of neuronal population. The status of neurogenesis in AD thus remains to be hotly debated, primarily due to the probable masking of this effect by AB toxicity on surrounding progenitor cells (Maurice A. Curtis, Faull et al., 2007). To this extent, there are several possibilities to explain the limited capacity of neurogenesis in disease states. Firstly the extent and rate of cell loss may be too great for neurogenesis to compensate. Secondly the neurons which are generated may fail to successfully integrate into the circuits. Lastly the microenvironment which the disease fosters may be toxic to new neurons. The latter may be resolved or alleviated by administration of growth factors and environment enrichment, two related factors which also result in altered neurogenesis (Jin, Peel et al., 2004; McKay, 2004). ConclusionAlthough the potential usefulness of neurogenesis in the adult human brain remains to be fully delineated, it is clear that after an insult such as ischemia or neurodegeneration, there exists in the brain an inherent mechanism to up regulate progenitor cell proliferation. By increasing SVZ NPC proliferation and migration towards sites of injury, as well as the successful integration of the progenitors into the damaged tissue, future research may improve mortality and quality of life of sufferers of neurodegerative disease. Whether progressive cell loss will continue to occur despite potential therapeutic intervention is a possible future direction of the field (Zhao, Deng, & Gage, 2008).
Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z., & Lindvall, O. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med, 8(9), 963-970. Barkho, B. Z., Song, H., Aimone, J. B., Smrt, R. D., Kuwabara, T., Nakashima, K., et al. (2006). Identification of Astrocyte-expressed Factors That Modulate Neural Stem/Progenitor Cell Differentiation. Stem Cells and Development, 15(3), 407-421. Becker, M., Lavie, V., & Solomon, B. (2007). Stimulation of endogenous neurogenesis by anti-EFRH immunization in a transgenic mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences, 104(5), 1691-1696. Cattaneo, E., & McKay, R. (1990). Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature, 347(6295), 762-765. Crespel, A., Rigau, V., Coubes, P., Rousset, M. C., de Bock, F., Okano, H., et al. (2005). Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiology of Disease, 19(3), 436-450. Curtis, M. A., Eriksson, P. S., & Faull, R. L. M. (2007). PROGENITOR CELLS AND ADULT NEUROGENESIS IN NEURODEGENERATIVE DISEASES AND INJURIES OF THE BASAL GANGLIA. Clinical and Experimental Pharmacology and Physiology, 34(5-6), 528-532. Curtis, M. A., Faull, R. L. M., & Eriksson, P. S. (2007). The effect of neurodegenerative diseases on the subventricular zone. Nat Rev Neurosci, 8(9), 712-723. Curtis, M. A., Penney, E. B., Pearson, J., Dragunow, M., Connor, B., & Faull, R. L. M. (2005). The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington's disease human brain. Neuroscience, 132(3), 777-788. Emsley, J. G., Mitchell, B. D., Kempermann, G., & Macklis, J. D. (2005). Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Progress in Neurobiology, 75(5), 321-341. Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A.-M., Nordborg, C., Peterson, D. A., et al. (1998). Neurogenesis in the adult human hippocampus. Nat Med, 4(11), 1313-1317. Gage, F. H. (2000). Mammalian Neural Stem Cells. Science, 287(5457), 1433. Gage, F. H. (2002). Neurogenesis in the Adult Brain. J. Neurosci., 22(3), 612-613. Gao, H., Liu, Y., Lu, S., Xiang, B., & Wang, C. (2006). A Reversible Middle Cerebral Artery Occlusion Model Using Intraluminal Balloon Technique in Monkeys. Journal of Stroke and Cerebrovascular Diseases, 15(5), 202-208. Haughey, N., Liu, D., Nath, A., Borchard, A., & Mattson, M. (2002). Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid β-peptide. NeuroMolecular Medicine, 1(2), 125-135. Haughey, N. J., Nath, A., Chan, S. L., Borchard, A. C., Rao, M. S., & Mattson, M. P. (2002). Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. Journal of Neurochemistry, 83(6), 1509-1524. Jin, K., Galvan, V., Xie, L., Mao, X. O., Gorostiza, O. F., Bredesen, D. E., et al. (2004). Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proceedings of the National Academy of Sciences, 101(36), 13363-13367. Jin, K., Peel, A. L., Mao, X. O., Xie, L., Cottrell, B. A., Henshall, D. C., et al. (2004). Increased hippocampal neurogenesis in Alzheimer's disease. Proceedings of the National Academy of Sciences, 101(1), 343-347. Jin, K., Wang, X., Xie, L., Mao, X. O., Zhu, W., Wang, Y., et al. (2006). Evidence for stroke-induced neurogenesis in the human brain. Proceedings of the National Academy of Sciences, 103(35), 13198-13202. Jin, K., Xie, L., Mao, X. O., & Greenberg, D. A. (2006). Alzheimer's disease drugs promote neurogenesis. Brain Research, 1085(1), 183-188. Lan Zhang, R., LeTourneau, Y., Gregg, S. R., Wang, Y., Toh, Y., Robin, A. M., et al. (2007). Neuroblast Division during Migration toward the Ischemic Striatum: A Study of Dynamic Migratory and Proliferative Characteristics of Neuroblasts from the Subventricular Zone. J. Neurosci., 27(12), 3157-3162. Lovell-Badge, R. (2001). The future for stem cell research. Nature, 414(6859), 88-91. Malcolm R. Alison, R. P. S. F. N. A. W. (2002). An introduction to stem cells. The Journal of Pathology, 197(4), 419-423. McKay, R. D. (2004). Stem cell biology and neurodegenerative disease. Philosophical Transactions of the Royal Society B: Biological Sciences, 359(1445), 851-856. Tattersfield, A. S., Croon, R. J., Liu, Y. W., Kells, A. P., Faull, R. L. M., & Connor, B. (2004). Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington's disease. Neuroscience, 127(2), 319-332. Temple, S. (2001). The development of neural stem cells. Nature, 414(6859), 112-117. Thored, P., Arvidsson, A., Cacci, E., Ahlenius, H., Kallur, T., Darsalia, V., et al. (2006). Persistent Production of Neurons from Adult Brain Stem Cells During Recovery after Stroke. Stem Cells, 24(3), 739-747. Thored, P., Wood, J., Arvidsson, A., Cammenga, J., Kokaia, Z., & Lindvall, O. (2007). Long-Term Neuroblast Migration Along Blood Vessels in an Area With Transient Angiogenesis and Increased Vascularization After Stroke. Stroke, 38(11), 3032-3039. Yamashita, T., Ninomiya, M., Hernandez Acosta, P., Garcia-Verdugo, J. M., Sunabori, T., Sakaguchi, M., et al. (2006). Subventricular Zone-Derived Neuroblasts Migrate and Differentiate into Mature Neurons in the Post-Stroke Adult Striatum. J. Neurosci., 26(24), 6627-6636. Yanamoto, H., Nagata, I., Niitsu, Y., Xue, J.-H., Zhang, Z., & Kikuchi, H. (2003). Evaluation of MCAO stroke models in normotensive rats: standardized neocortical infarction by the 3VO technique. Experimental Neurology, 182(2), 261-274. Zhao, C., Deng, W., & Gage, F. H. (2008). Mechanisms and Functional Implications of Adult Neurogenesis. Cell, 132(4), 645-660. Suggested Reading from Inquiries Journal
Inquiries Journal provides undergraduate and graduate students around the world a platform for the wide dissemination of academic work over a range of core disciplines. Representing the work of students from hundreds of institutions around the globe, Inquiries Journal's large database of academic articles is completely free. Learn more | Blog | Submit Latest in Biology |