Carbon Capture and Storage as a Method to Mitigate Climate Change
By
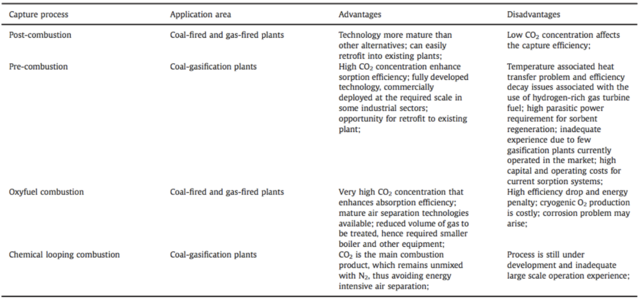
2017, Vol. 9 No. 03 | pg. 1/2 | » AbstractThrough increased industry practices, land degradation, and burning of fossil fuels the anthropogenic sources of CO2 have increased creating global concern due to the adverse impacts of climate change. Carbon dioxide capture and storage (CCS) technologies have been widely studied as a viable method to reduce carbon emissions. This paper portrays a holistic overview of the physical methods as well as the biological methods of carbon sequestration in order to transition towards a net carbon neutral economy. The physical processes discussed are carbon capture, separation and storage, whereas the biological sequestration processes discussed include afforestation, ocean storage and algal use. Carbon capture usually occurs through chemical processes during the pre-combustion, post-combustion and oxyfuel combustion stages. (Leung et al., 2014) It was found that the most mature and viable technologies for carbon capture was post combustion because it can be retrofitted in older power plants. (Leung et al., 2014) Adsorption was found the best technology to separate the carbon because it is mature, simple and relatively low cost. (Aaron et al., 2005) Lastly, depleted oil and gas reservoirs are the most common geological formations for carbon storage. (Leung et al., 2014) From the biological processes discussed, microalgae was the most promising due to their fast growth and high photosynthetic activity. (Singh et al., 2013) There are many limitations for many of the technologies specifically lack of funding and monetary incentive to reduce carbon dioxide emissions as well as the maturity of the research. IntroductionIncreased burning of fossil fuels, deforestation, land degradation and various industry practices have resulted in high atmospheric CO2 levels creating global concern because of its implications for climate change. These anthropogenic sources have increased global emissions of CO2 by 48% more than two decades ago thus accelerating the greenhouse gas effect and increasing the overall temperature of the planet by 0.8oC in 2013 (Farrelly et al., 2013)(Leung et al., 2014) This is detrimental to the environment as it leads to the melting of ice caps, increased sea level rise and extreme weather conditions. (Farrelly et al., 2013) Recently, global leaders committed to keep the overall temperature rise of the planet to under 2oC at the COP21 in Paris promising to mitigate climate change by moving toward a carbon free future. It is clear that in order to tackle the challenge many approaches have to be implemented together such as “energy efficiency, alternative energy sources, energy conservation as well as carbon capture and sequestration.” (Litynski et al., 2006) Carbon sequestration strategies can be a clear path in mitigating climate change as well as neutralizing the excess CO2 in the atmosphere emitted through anthropogenic sources. (Singh et al., 2013) There are several mitigation strategies for CO2 that have been studied and are currently being used in various pilot projects further increasing the incentive to study this field. This paper means to give a holistic overview of carbon sequestration as a method to mitigate climate change by focusing specifically on emerging carbon capture and storage technologies and biological mitigation including afforestation, ocean sequestration and microalgae use. Overview of the Carbon CycleThe most recent studies on the geochemical cycle of carbon show that atmospheric CO2 in the last 106 years is regulated by the carbon cycle which includes “silicate weathering, carbonate precipitation, carbonate metamorphism, mantle CO2 degassing, oxidative weathering and burial of organic carbon.” (Farrelly et al., 2013) In order to understand new and emerging methods in carbon sequestration it is crucial to view the natural regulation of carbon on the planet. Carbon is exchanged and recycled among natural reservoirs and processes, which occur at various rates ranging from short-term to long-term. (NOAA) Short-term processes are daily and seasonal cycles where carbon is released into the atmosphere by respiration from plants and animals as well as decomposition of biomass. (NOAA) On a time scale, which spans decades and centuries, carbon dioxide levels are fluctuated by surface and deep ocean mixing whereas on a geologic time scale carbon is stored as carbonate rock or released through volcanoes which produce CO2 gas from the rocks stored underground. (NOAA) Around 123GtC/year is stored in biomass and released through respiration at 60 GtC/year. (Farrelly et al., 2013) Furthermore a release of carbon through decaying biomass at 60 GtC/year gives a net sequestration amount of carbon at 3 GtC/year in soils. (Farrelly et al., 2013) Similarly in oceans there is a net 2 GtC/year of carbon sequestration from the absorption of photosynthetic organisms and release by decay and respiration. (Farrelly et al., 2013) Volcanoes release a very small amount of carbon (0.13 GtC/year), which is extremely small compared to the amount of carbon anthropogenic sources have released in the past 40 years estimated to be around 9 GtC/yr. (Farrelly et al., 2013) Due to these major issues and global leaders struggling to manage and mitigate climate change, it is crucial to study mitigation methods both physical and biological that can store and sequester carbon indefinitely or in the short-term.Physical Carbon Sequestration MethodsCarbon Capture and Storage TechnologiesCarbon capture and storage (CCS) includes a large range of technologies that involve different processes for CO2 “capture, separation, transport, storage and monitoring.” (Leung et al., 2014) They are usually defined as the “removal of CO2 that would otherwise be emitted in the atmosphere.” (Farrelly et al., 2013) CCS can reduce emissions by 80%-95% from very large point source emission sources such as industry power plants specifically for the cement or energy industries. (Leung et al., 2014) Using this approach the carbon is captured pre-combustion and post-combustion, “separated from the sorbent, stored or reutilized industrially.” (Leung et al., 2014) The most used and applicable reservoirs for these types of technologies are depleted oil and gas reservoirs, un-minerable coal beds as well as deep saline formations. (Farrelly et al., 2014) Carbon CaptureDue to the fact that carbon dioxide is formed during various combustion processes, specific techniques must be put into place to choose the appropriate removal. The main CO2 systems that are associated with these different processes include pre-combustion, post-combustion, oxyfuel combustion. Figure 1 gives an overview of the advantages and disadvantages of each of the technologies and the area in which they are applied to. Figure 1. The advantages and disadvantages of the different technologies used for carbon capture (Leung et al., 2014). Pre-CombustionPre-combustion capture normally occurs for coal or natural gas, where the fuel is pre treated in order release the minimal amount of carbon dioxide. (Leung et al., 2014) Coal undergoes the gasification process where it is pretreated in a gasifier under low oxygen levels creating carbon monoxide and hydrogen gas. (Leung et al., 2014) The CO will then be converted to carbon dioxide through a water-gas shift reaction and subsequently captured. This technique can be applied to the Integrated Gasification Combined Cycle power plants that use coal as fuel. Furthermore, natural gas can also be treated through a pre-combustion process as it mainly contains methane. It can be reformed to syngas that contains both carbon monoxide and hydrogen gas using the reaction below. (Leung et al., 2014) CH4 + H2O à CO + H2 (Leung et al., 2014) Through this pre-combustion process the H2 from the syngas can be used as clean energy or in other applications. (Muradov et al., 2008) Figure 2. Shows the pre-combustion process in detail where the coal and the natural gas are turned to syngas through gasification and the hydrogen gas is then used in power plants as a form of renewable energy. Figure 2. The gasification process of coal and natural gas in pre-combustion capture technologies (Muradov et al., 2008) Post-CombustionPost-combustion carbon capture (PCC) technologies are the most preferred worldwide as they can easily be retrofitted into old power plant systems. They usually remove the carbon dioxide form the flue gas after the combustion takes place (hence the name). At the moment, this process is the only industrial CO2 capture used on a full commercial scale. (Liang et al., 2015) The PCC process uses a chemical adsorbent, where the raw gas enters through an inlet separator; its liquid and solid particles are removed and then the gas flows from the bottom of the “absorber upwards against a stream of lean solution.” (Liang et al., 2015) “The carbon dioxide in the flue gas is absorbed and the treated flue gas leaves the top of the absorber.” (Liang et al., 2015) In the past decade a lot of progress has been made in using specific solvents for this process, which provide almost complete absorption and desorption of CO2 enhancing the effectiveness of the technology. (Liang et al., 2015) Oxyfuel CombustionOxyfuel combustion is also used in various power plants and it involves burning the fuel with “nearly pure oxygen instead of air.” (Stranger et al., 2015) The main purpose is to generate a flue gas with a very high concentration of CO2 and water vapour in order to then separate the carbon dioxide through dehydration and low temperature purification processes. (Stranger et al., 2015) The major units in oxyfuel combustion for power generation are the Air Separation Unit which is used for the oxygen production, the boiler or gas turbine used for generation of heat and fuel combustion, the flue gas processing unit used to clean the gas and the CO2 processing unit used for the final purification and transport of the CO2. (Stranger et al., 2015) Because oxygen is used instead of air, nitrogen is controlled and does not need to be separated in the later stages, which is one of the major advantages of this process. (Leung et al., 2014) The major composition of the flue gas is carbon dioxide, water particulates and SO2 that can be easily removed through existing technology. (Leung et al., 2014) At the moment, there are only a few pilot projects and no full scale technological implementation of this process which leads to the comparison of the three capture technologies as seen in Figure 1 Leung et al., have reviewed and concluded that the post-combustion technology is the most easily implementable yet it has a very low CO2 capture efficiency. Pre-combustion is highly efficient yet it is costly and there is inadequate experience due to very little gasification plants in the market and finally the oxyfuel combustion process has a high cost due to the need for cryogenic O2, yet it is very high in absorption efficiency of CO2 as well as there are mature separation techniques available on the market. Carbon SeparationOnce the carbon is captured, various technologies and methodologies exist to separate it from the source in order to have it ready for storage or transportation. Wet scrubbers, sorbents, membranes, cryogenics adsorption as well as various other methods exist in order to complete this process. (Leung et al., 2014) Due to the many methodologies, the main focus of this section will be on absorption, adsorption, and membrane separation. AbsorptionAs was previously mentioned in the oxyfuel combustion section, carbon separation by absorption requires a “solvent to dissolve CO2 but not oxygen, nitrogen, gas or any other components of the flue gas stream.” (Aaron et al., 2005) The most optimal conditions for absorption are low temperature, high pressure as well as it must occur after electrostatic precipitation. (Aaron et al., 2005) This needs to occur because the compounds found in fossil fuel sources such as fly ash, SOx and NOx, degrade many solvents. In these absorption processes, once the flue gas is clear of CO2 it is emitted back into the atmosphere or used in other applications such as chemical production. Aaron et al., propose that an effective and economical solvent for this process is monoethanolamine (MEA) because it can selectively absorb CO2 as well as be easily regenerated. Furthermore, the team studied solid absorbents such as calcium and lithium hydroxides that need very high temperatures but can absorb the CO2 very quickly. Other sorbents that can be used are diethanolamine (DEA) and potassium carbonate. (Leung et al., 2014) This is one of the most mature methods of separation due to its high efficiency and relatively low cost, yet it could pose other environmental problems that are related to the degradation of the sorbent itself. (Leung et al., 2014) AdsorptionAdsorption differs from absorption because it is a heterogeneous process that does not require the dissolution of CO2 into a solvent. (Aaron et al., 2005) During adsorption the CO2 molecules are trapped and attracted by the surface groups of the sorbent, specifically zeolites, activated carbon and molecular sieves. (Aaron et al., 2005) Figure 3. Schematic diagram of a single chamber adsorption system (Aaron et al., 2005).
Membrane SeparationVarious types of membranes are used in order to separate CO2 from the flue gas. It is one of the simpler methods, which gives an advantage over other technologies. However, membranes that are selective are not very permeable and permeable membranes are not very selective. (Aaron et al., 2005) Brunetti et al., have summarized the criteria needed for membranes to be used on a large-scale basis. They propose that it has to have high carbon dioxide permeability and selectivity, it has to be thermally and chemically resistant, plastic, aging resistant, cost effective and ability to be manufactured at low cost. (Brunetti et al., 2010) Polyamides, facilitated transport membranes, mixed matrix membranes and carbon molecular sieves are the most promising technologies with polymer-based membranes being the most highly studied. (Brunetti et al., 2010) Figure 3 shows the typical single chamber adsorption system where the flue gases enter after being cooled which brings down the temperature to 30oC. It is then pressurized by flue gas with compressors to create a highly efficient CO2 adsorption. (Aaron et al., 2005) The first chamber is pressurized (capturing the carbon dioxide) and the second is depressurized allowing the carbon dioxide to be collected and transported elsewhere. Recently, Vishwajeet et al., have proposed the sequestration of carbon dioxide with red mud, which is a hazardous waste created from the aluminum industry and it also acts as a powerful adsorbent. This decreases the impact of waste on the environment and can be used as a cheap alternative to other adsorbents. Figure 4. Schematic diagram of a membrane separation tube. (Aaron et al., 2005).
Figure 4 depicts a membrane separation tube where the flue gases enter the separation tank and the carbon dioxide diffuses across the membrane. (Aaron et al., 2005) The CO2 is pulled across the membrane because of a pressure difference, which was at first initiated by a vacuum. (Aaron et al., 2005) It is then separated from the rest of the flue gas components and ready for storage or transport. Physical Carbon StoragePhysical carbon storage methodologies and technologies usually store CO2 into different geological formations such as depleted oil and gas reservoirs, deep ocean storage, saline aquifers or others that are not used in any other practical way. (Leung et al., 2014) At the present moment, it is the most viable option for storage of large quantities of CO2, yet the sites have to be very carefully selected. The requirements usually consist of finding the appropriate “porosity, thickness and permeability of the reservoir rock, a cap rock with good sealing capabilities and stable geological environment.” (Leung et al., 2014) Using geological media as storage can also have its limitations due to the fact that there is a potential for leakage, which has to all be considered when choosing the best way to store the carbon. Leung et al, and Bachu have described that the criteria for the process of selecting a suitable geological site has to include “tectonic setting and geology of the basin, geothermal regime, hydrology of formation waters, hydrocarbon potential and basin maturity.” (Leung et al., 2014) (Bachu, 2013) Economic barriers also have to be overcome, making the most feasible geological storages to be depleted oil and gas reservoirs, saline aquifers and unminable coal beds. Depleted Oil and Gas ReservoirsThe carbon dioxide can be injected within the depleted (or nearly depleted) oil and gas reservoirs, to extract the residual oil and gasses as well as store the CO2 permanently. (Leung et al., 2014) Injection technologies for mitigation are very mature with studies on various aspects such as enhanced oil recovery, geochemical modeling, and leakage, risk assessment as well as migration simulation. (Leung et al., 2014) Furthermore there is also a large economic incentive for oil/gas industries to use the reservoirs making this storage method one of the most viable. At the moment there are many ongoing storage projects, such as a Canadian oil reservoir having the ability to store 30 million tonnes of CO2 from a gasification plant in America, transported via pipeline. (Leung et al., 2014) Saline AquifersSaline aquifers are also being studied as a viable method to store large amounts of CO2 because of their low commercial value. Saline aquifers are in many widespread areas, and often occur at 700-1000m below the ground hosting “high salinity formation brines.” (Leung et al., 2014) In order to store the CO2 within the aquifers it would need to be trapped at a major point source as discussed in the previous sections, and transported to be injected within the saline aquifers. (Lemiux, 2011) The trapping mechanisms that occur in these aquifers can be different. One way to trap is through “hydrodynamic residual,” where the undissolved CO2 is trapped “by overlying low-rock permeability caprock, and is gradually dispersed. The CO2 then takes the place of the water in the rock displacing it from the pore and the “whole rock volume retains a residual saturation of CO2. (Leung et al., 2014)Continued on Next Page » Suggested Reading from Inquiries Journal
Inquiries Journal provides undergraduate and graduate students around the world a platform for the wide dissemination of academic work over a range of core disciplines. Representing the work of students from hundreds of institutions around the globe, Inquiries Journal's large database of academic articles is completely free. Learn more | Blog | Submit Latest in Environmental Studies |